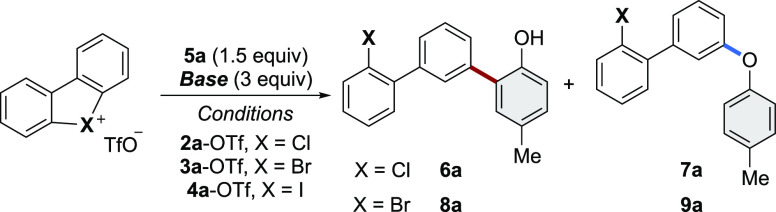
Table 1. Reactivity of Cyclic Diaryl Hypervalent λ3-Reagents.
| yieldsb |
||||
|---|---|---|---|---|
| entry | X(III)-OTf | conditionsa | C–C | C–O |
| 1 | 4a-OTf | Cs2CO3; CHCI3 | no conversion | |
| 2 | 3a-OTf | 15% | 70% | |
| 3 | 2a-OTf | 35% | 35% | |
| 4 | 4a-OTf | K2CO3; CHCI3 | no conversion | |
| 5 | 3a-OTf | 26% | 9% | |
| 6 | 2a-OTf | 49% | traces | |
| 7 | 4a-OTf | K2CO3; H2O | no conversion | |
| 8 | 3a-OTf | no conversion | ||
| 9 | 2a-OTf | 13% | 60% | |
Conditions: 0.1 mmol of X(III)-OTf reagent (4a-OTf, 3a-Tf, 2a-OTf), 0.15 mmol of 5a, and 0.3 mmol of Cs2CO3 or K2CO3 in 1 mL of CHCl3 or H2O at room temperature for 16h.
Isolated yields.