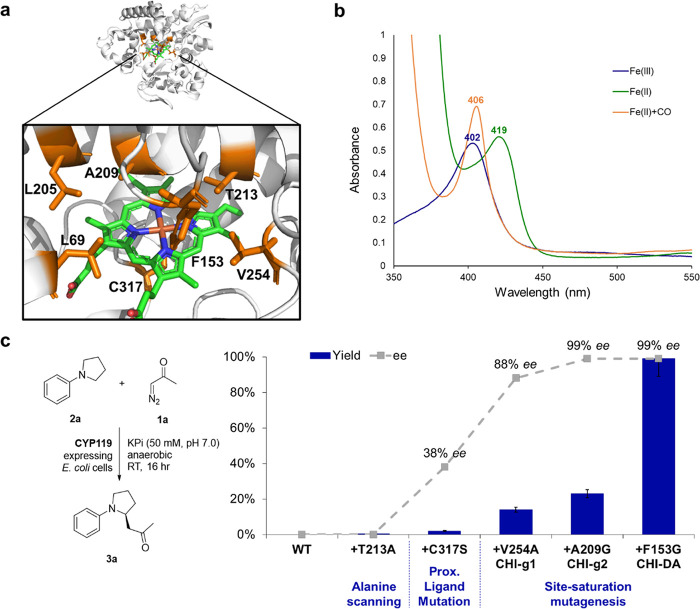
Figure 3.
Structure, spectral properties, and directed evolution of CYP119 catalysts for enantioselective C–H functionalization of N-phenylpyrrolidine with diazoacetone. (a) X-ray crystal structure of CYP119 from S. solfataricus (PDB 1IO7 61). Amino acid residues targeted for mutagenesis are highlighted in orange, and the heme cofactor is shown in green. (b) UV–vis absorption spectra for CYP119 (T213A, C317S) in its ferric state (Fe(III), blue), ferrous state (Fe(II), green), and CO-bound form (Fe(II)+CO, orange). (c) Directed evolution of CYP119 catalysts for enantioselective C–H functionalization of N-phenylpyrrolidine (2a) with diazoacetone (1a). Yields and % ee as determined under standard reaction conditions with diazoacetone (Table 1).