Figure 1. Affinities of Env proteins to CH31.
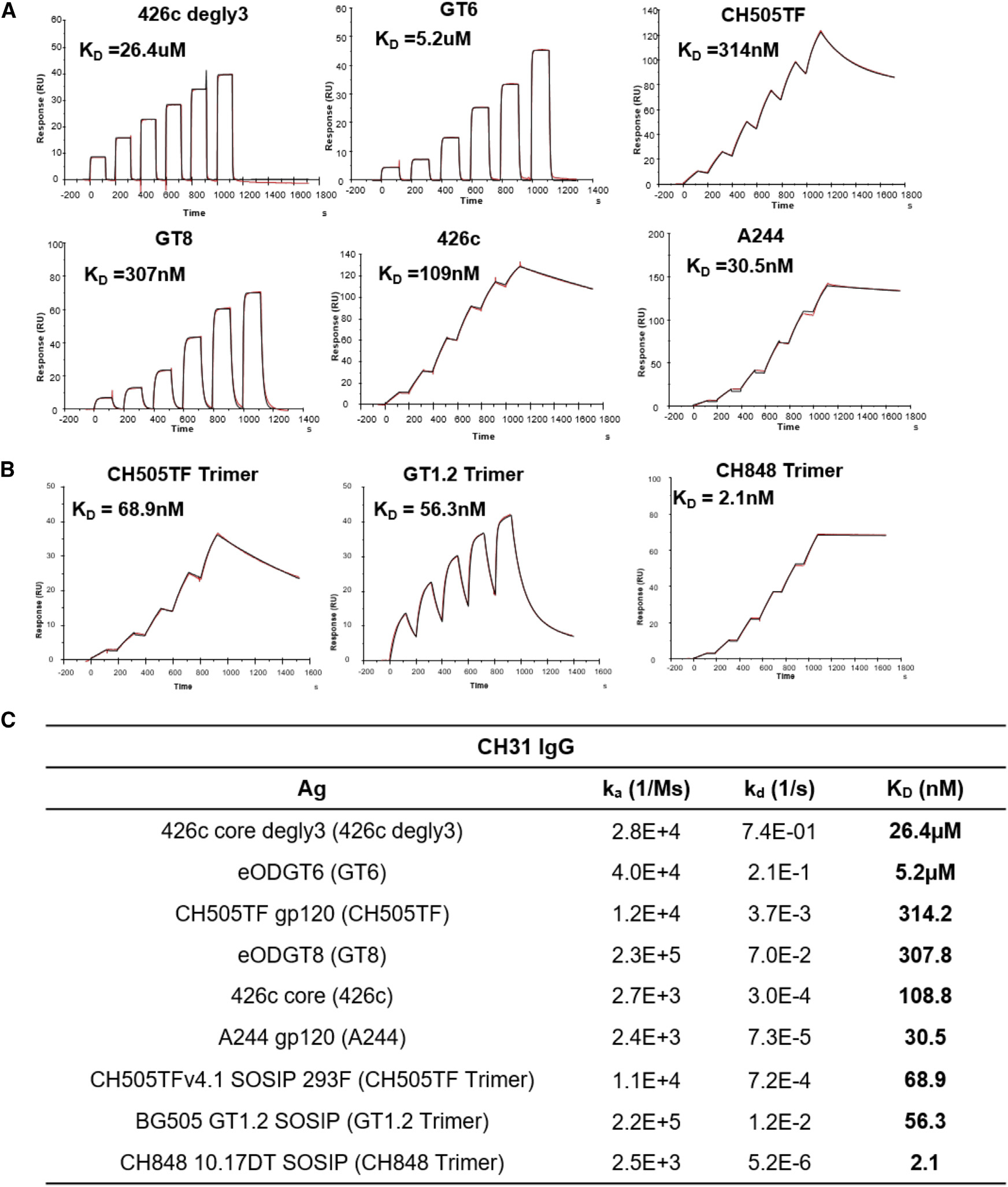
(A) Surface plasmon resonance (SPR) single-cycle kinetic binding profiles and affinities of Env proteins (core and gp120) to the CD4-binding site bnAb CH31 IgG. Six sequential injections of each antigen at concentrations ranging from 25 to 5,000 nM were flowed over CH31 IgG mAbs. Curve-fitting analyses were performed using either a 1:1 Langmuir model or the heterogeneous ligand model.
(B) Single-cycle kinetic binding profiles and affinities of CH31 IgG Fab (30–1,500 nM) against biotinylated GT1.2, CH505TF, or CH848 trimers immobilized to a streptavidin-coated sensor chip. The heterogeneous ligand model was used for fitting CH31 Fab against GT1.2 trimer, and the 1:1 Langmuir model was used for fitting against both the CH505TF and CH848 trimers.
(C) Binding affinities (KD) and kinetic rate constants (association and dissociation rates) of each protein to either CH31 IgG mAb or Fab. Binding curves and values for the kinetic rates and affinities are representative of at least two independent measurements.
