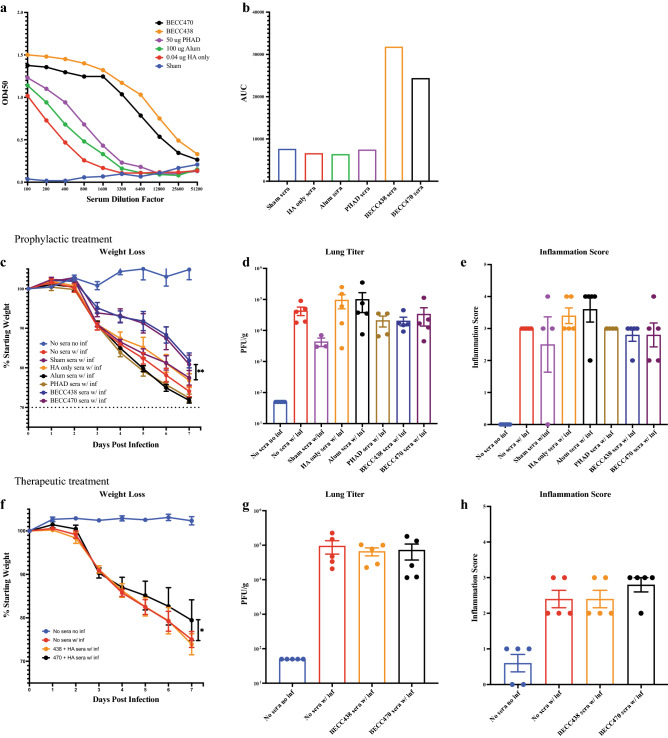
Figure 3.
Protection from homologous H1N1 challenge with prophylactic or therapeutic passive transfer of vaccination sera. (a) Pooled, day-28 serum H1-specific ELISA total IgG of young adult BALB/c mice vaccinated with 0.04 µg Cal/09 rHA protein in combination with 100 µg alum, 50 µg PHAD 50 µg BECC438 or 50 µg BECC470 adjuvant in prime-boost schedule (mean ± SEM). (b) AUC of pooled, pre-infection total IgG antibody titer curve (mean). (c) 7-day weight loss (mean ± SEM) of BALB/c mice IP-injected with 100 µL vaccination sera (by group) and infected 2 h later with 3200 PFU NL/09 (**p = 0.008). (d) Virus titer (mean + SEM) of mouse lung homogenate in (c), 7-days post-infection. (e) Pathology inflammation scoring (mean ± SEM) of lung histology slides of mice in (c), 7-days post-infection. (f) 7-day weight loss (mean ± SEM) of BALB/c mice infected with 3200 PFU NL/09 and IP-injected with 100 µL vaccination sera (by group) at day 1 and day 3 post-infection (*p = 0.0288). (g) Virus titer (mean ± SEM) of mouse lung homogenate in (f), 7-days post-infection. (h) Pathology inflammation scoring (mean ± SEM) of mouse lung histology slides in (f), 7-days post-infection.