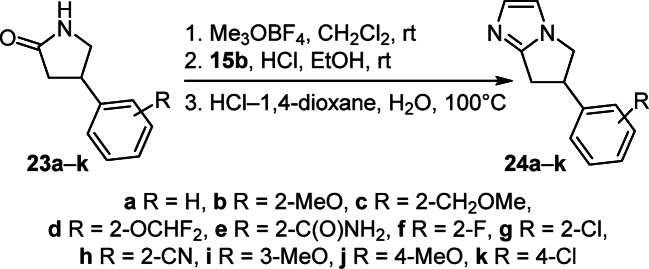Abstract
The review summarizes and systematizes the literature data on the synthesis and some aspects of application of pyrrolo[1,2-a]imidazoles. Synthetic approaches are grouped according to the degree of saturation of the product pyrroloimidazole ring. The bibliography of the review includes 110 sources over the last 15 years.
Keywords: hydrogenated pyrrolo[1,2-a]imidazoles; cyclization; cycloaddition; cyclocondensation
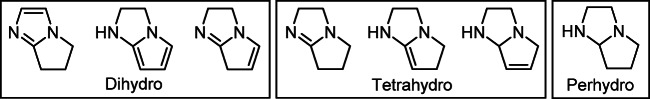
Partially and fully hydrogenated pyrrolo[1,2-a]imidazole heterocyclic systems are valuable synthetic blocks and have a wide spectrum of biological activity. Depending on the degree of saturation, dihydropyrrolo[1,2-a]imidazoles (structures I–III, Fig. 1), tetrahydropyrrolo[1,2-a]imidazoles (structures IV–VI), and perhydropyrrolo[1,2-a]imidazoles (structure VII) may be distinguished.
Figure 1.
The structure types of pyrrolo[1,2-a]imidazoles I–VII.
To date, 6,7-dihydro-5H-pyrrolo[1,2-a]imidazoles I and fully hydrogenated pyrrolo[1,2-a]imidazoles VII are the most studied due to the discovery among them of dimiracetam (Fig. 2), a nootropic drug of the racetam family,1 as well as of compound VIII, a potent α1A-adrenergic receptor partial agonist with good selectivity for α1B, α1D, and α2A receptor subtypes.2
Figure 2.

The structures of the nootropic drug dimiracetam and the α1A-adrenergic receptor partial agonist VIII.
An analysis of literature sources indicates that interest in these compounds appeared as early as the 1960s,3,4 while advances in the chemistry of pyrroloimidazoles were first summarized in a 1995 review.5 Research over the next two decades, which relate to synthetic methods, chemical transformations, and biological activity, was partially summarized in concise reviews in 20086 and 2022.7 Several examples of annulation of a pyrroloimidazole scaffold are described in a microreview published in 2016.8 Unfortunately, these publications do not provide a complete picture of the advances in the chemistry of pyrroloimidazoles over the past 15 years. In addition, the questions of the practical application of pyrrolo[1,2-a]-imidazoles and their powerful biological potential remained outside of their scope.
For this reason, it seemed to us reasonable to comprehensively generalize and systematize the array of data on the synthesis methods, use in organic synthesis, and medical and biological studies of hydrogenated pyrrolo[1,2-a]imidazoles.
THE SYNTHESIS METHODS OF DIHYDROPYRROLO[1,2-a]IMIDAZOLES
Annulation of the imidazole ring to the pyrrole ring
The pyrrolo[1,2-a]imidazole scaffold was formed by the condensation of aminopyrrolines with halocarbonyl compounds, which, however, did not result in high yields of the target products. In particular, the reaction of 3,4-dihydro-2H-pyrrol-5-amine (1) with 2-bromo ketones 2а–n in EtOAc at room temperature led to 3-substituted pyrrolo[1,2-a]imidazole hydrobromides 3а–n (no yields given).9 At the same time, in the case of pyridyl bromo ketone 2о, heating in DMF in the presence of Na2CO3 gave isomeric 2-substituted pyrrolo[1,2-a]imidazole 4 in only 14% yield (Scheme 1).10

Scheme 1
Treatment of iminopyrrolidines 5а–d with α-phenacyl bromides 2р–r in MeCN followed by heating in acetic anhydride led to the formation of pyrrolo[1,2-a]imidazolium bromides 6a–е. The latter were smoothly partially reduced with NaBH4 in DMF to afford tetrahydro-1Hpyrrolo[ 1,2-a]imidazoles 7a–е (Scheme 2).11

Scheme 2
Intramolecular cyclization of 2-(2-oxopyrrolidin-1-yl)-acetamide (8) by the action of POBr3 or POCl3 gave 2-halosubstituted pyrrolo[1,2-a]imidazoles 9a,b (Scheme 3).12 The microwave modification of this method made it possible to synthesize compound 9a in 82% yield.13
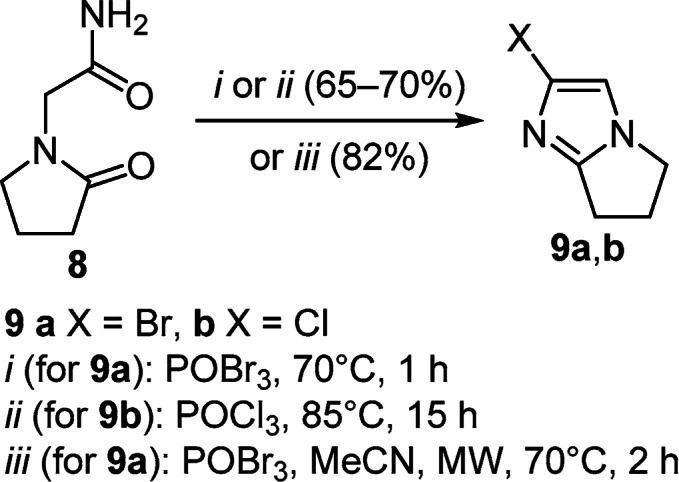
Scheme 3
Cyclocondensation of 2-methoxypyrroline 10 with aminoacetonitrile hydrochloride (11) in i-PrOH under reflux led to 6,7-dihydro-5H-pyrrolo[1,2-a]imidazol- 3-amine hydrochloride (12),14 while cyclocondensation of compound 10 with amino ketone hydrochlorides 13а–е resulted in the formation of 3-arylpyrrolo[1,2-a]imidazoles 14a–e (no yields given, Scheme 4).15

Scheme 4
The reaction of pyrroline 10 with 2,2-dimethoxyethanamine (15a) in the CH2Cl2–MeOH system gave aminopyrroline 16, the cyclization of which in formic acid gave the simplest 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole (17a) (Scheme 5).16,17 Its 6,6-dimethyl analog 17b was obtained in quantitative yield by similar transformations from 3,3-dimethyl-5(methylsulfanyl)-3,4-dihydro-2H-pyrrole hydroiodide (18) and amine 15а (Scheme 6).16

Scheme 5
Scheme 6
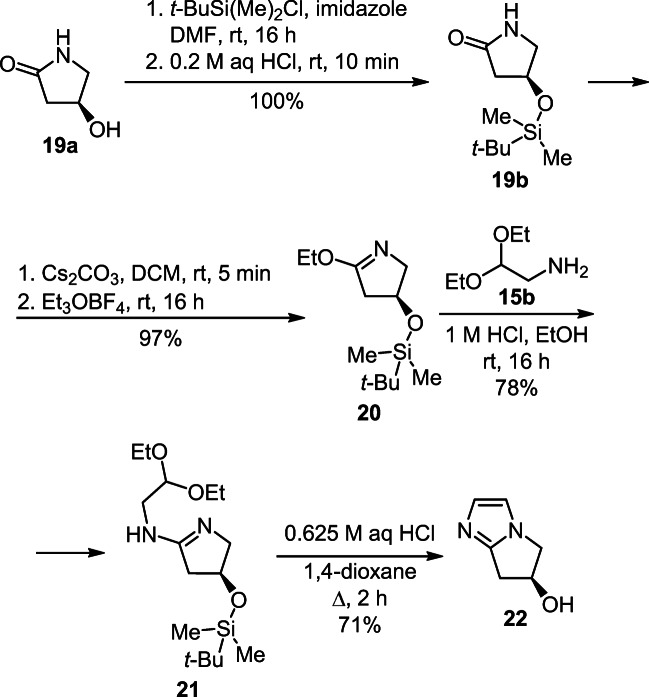
A four-step synthesis of (S)-6,7-dihydro-5H-pyrrolo[1,2-a]-imidazol-6-ol (22) was developed based on silylation of 4-hydroxypyrrolidin-2-one (19a), transformation of the obtained derivative 19b into cyclic imidate 20, its subsequent amination with aminoacetal 15b, and cyclization of the resulting amidine 21 (Scheme 7).18,19
Scheme 7
6-Aryl-6,7-dihydro-5H-pyrrolo[1,2-a]imidazoles 24a–k were synthesized from γ-lactams 23a–k by O-methylation with Meerwein's reagent followed by amidination with aminoethyl diethyl acetal 15b and cyclization under acidic conditions (no yields given, Scheme 8).20
Scheme 8
A patent21 describes a method for the synthesis of 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole-2-carboxylate (27) by the reaction of ethyl 3-(pyrrolidin-1-yl)acrylate (25) with 4-nitrobenzenediazonium tetrafluoroborate (26) in MeCN at room temperature followed by cyclization of intermediate hydrazone A in the presence of a base (no yield given, Scheme 9).

Scheme 9
The synthesis of dihydropyrrolo[1,2-a]imidazole-2,3-dicarboxylate 30 is based on the reaction of pyrrolidine 28а with acetylenedicarboxylate 29а and involves the one-pot formation of four C–N bonds, which is realized via the steps of hydroamination/azidation/cyclization (Scheme 10).22

Scheme 10
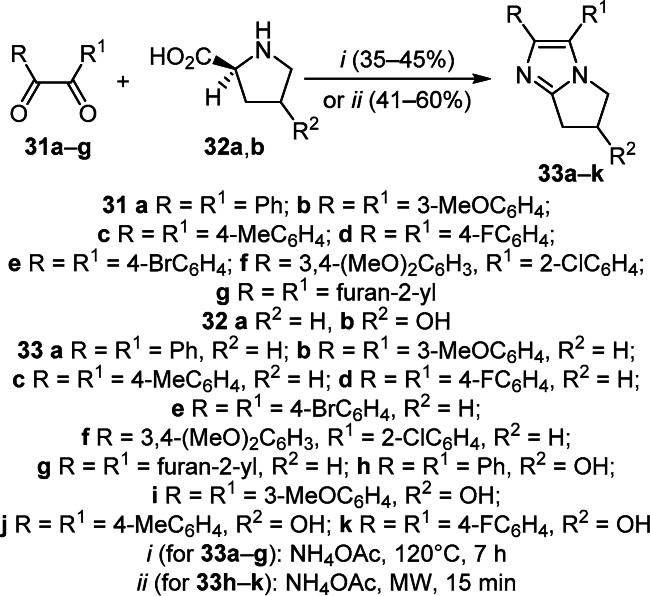
To obtain 2,3-disubstituted pyrrolo[1,2-а]imidazoles 33а–g, one-pot three-component condensation of aryl 1,2-diketones 31а–g, L-proline (32а), and NH4OAc in a ratio of 1:1:1 was employed. The microwave modification of this method proved to be suitable for 3-hydroxy-L-proline (32b) and was employed to access pyrrolo[1,2-а]imidazol-6-ols 33h–k (Scheme 11).23
Scheme 11
The one-pot synthesis of 6,7-dihydro-5H-pyrrolo[1,2-a]-imidazoles 35a–f was developed on the basis of a cascade of [3+2] cycloaddition and oxidative aromatization reactions. The reaction of phenacyl azides 34a–f with L-proline (32a) in PhMe under reflux led to the target products 35а–f in high yields (Scheme 12).24
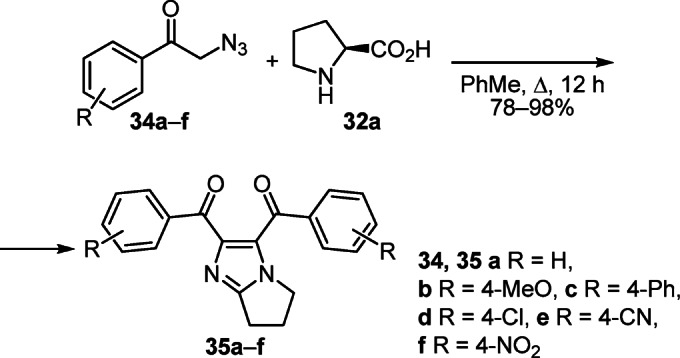
Scheme 12
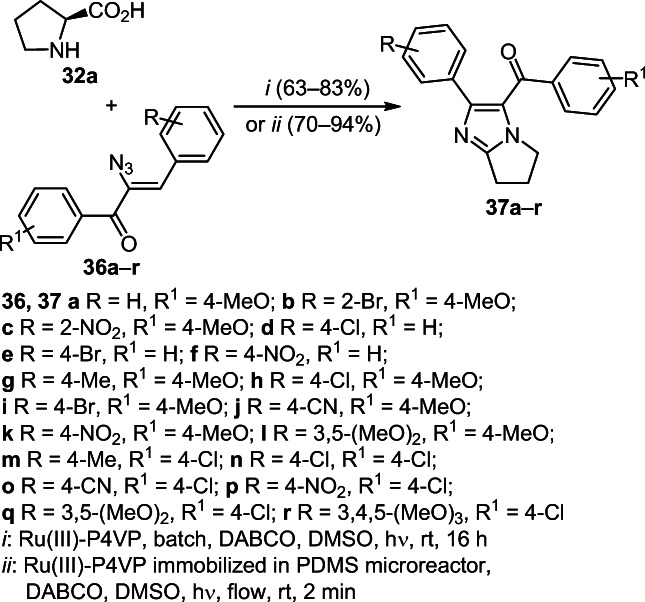
Photocatalytic annulation with decarboxylation of L-proline (32а) with α-azidochalcones 36a–r in the presence of ruthenium(III) polyvinylpyridine complex (Ru(III)-P4VP) proved to be an effective method for constructing pyrrolo[1,2-a]imidazoles 37a–r. A new environmentally benign and efficient version of this method, developed for a continuous flow in a polydimethylsiloxane (PDMS) microreactor with an immobilized Ru(III)-P4VP complex initiated by irradiation with visible light, provides excellent yields of target products 37a–r in a sufficiently short reaction time (Scheme 13).25
Scheme 13
Annulation of the pyrrole ring to the imidazole ring
The condensation of 1Н-imidazole (38) with acrolein (39) provides a simple and convenient method for the preparation of 6,7-dihydro-5H-pyrrolo[1,2-a]imidazol-7-ol (40),26–29 which has found application as a phosphorus-free catalyst in the Morita–Baylis–Hillman reaction,27 and is also a substrate in the synthesis of ionic liquids (Scheme 14).30
Scheme 14
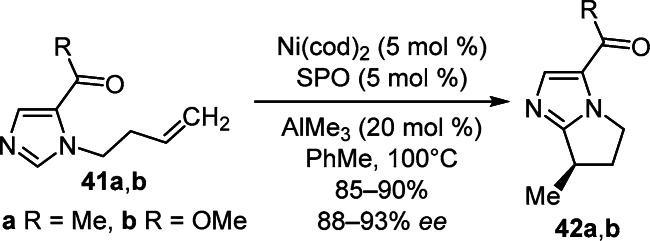
An enantioselective intramolecular cyclization of N-alkenyl-substituted imidazoles 41a,b catalyzed by the Ni–Al bimetallic system was developed for the synthesis of pyrrolo[1,2-a]imidazoles 42a,b possessing a β-stereocenter. It is important to note the decisive role of the SPO chiral phosphorus-containing ligand for stereocontrol of the process (Scheme 15).31
Scheme 15
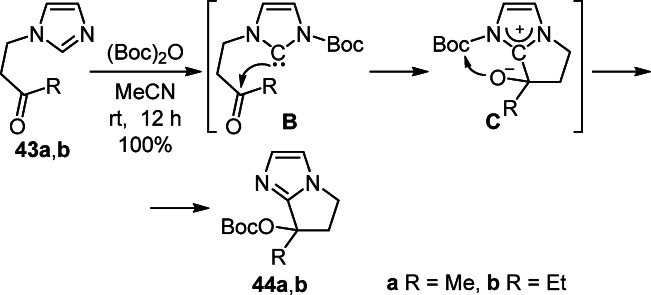
A simple method for the synthesis of pyrrolo[1,2-a]- imidazoles 44a,b is based on the intramolecular cyclization of ketones 43a,b by the action of (Boc)2O in MeCN at room temperature. An important feature of this transformation is the participation of the N-acylimidazolium carbene B and the transfer of the Boc group from the nitrogen atom to the oxygen nucleophilic center of intermediate C (Scheme 16).32
Scheme 16
A palladium-catalyzed cascade reaction of imidazolyl-1,6-diene 45 was described. It proceeds via a Heck-type carbopalladation reaction followed by C–H activation of the added N-heterocycle and leads to the formation of 1H-spiro[pyridine-3,6'-pyrrolo[1,2-a]imidazole] 46 (Scheme 17).33

Scheme 17
Intramolecular [2+2] photocyclization of 1-(1Н-imidazol-1-yl)-2-phenylprop-2-en-1-one (47) initiated by blue visible light in the presence of an iridium(III) complex led to pyrroloimidazolone 48 as a mixture of regioisomers with a combined yield of 21% (Scheme 18).34
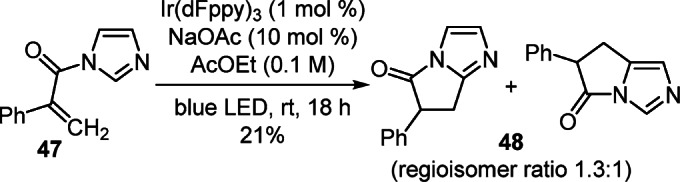
Scheme 18
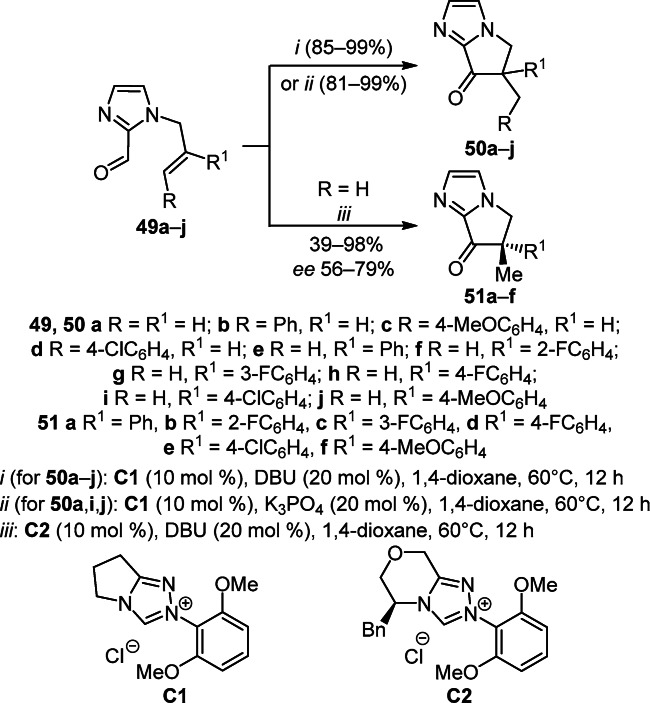
Intramolecular hydroacylation of N-allylimidazole-2-carboxaldehydes 49a–j catalyzed by N-heterocyclic carbene (NHC) catalyst C1 was used to obtain 5,6-dihydro-7H-pyrrolo[1,2-а]imidazol-7-ones 50a–j. In turn, the enantioselective hydroacylation of N-allylimidazole-2-carboxaldehydes 49e,g–j catalyzed by chiral NHC catalyst C2 proceeded with the formation of derivatives 51а,c–f in high yields (81–98%) with moderate enantioselectivity (ee 67–79%), although hydroacylation of aldehyde 49f resulted in the formation of target product 51b in just 39% yield and 56% ee (Scheme 19).35
Scheme 19
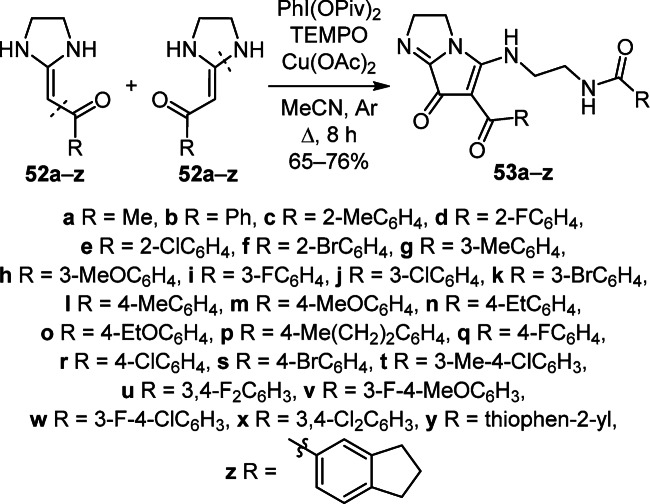
A new efficient method for the construction of functionalized 2H-pyrrolo[1,2-a]imidazol-7-ones 53a–z was implemented via a cascade reaction involving oxidative dimerization, ring opening, substitution, addition, and allyl oxidation by heating a mixture of ketenaminals 52a–z with TEMPO, PhI(OPiv)2, and Cu(OAc)2 under reflux in MeCN (Scheme 20).36
Scheme 20
Other methods
The reaction of methyl 4-chlorobutanimidate hydrochloride (54) with 2,2-dimethoxyethanamine (15a) led to 4-chloro-N-(2,2-dimethoxyethyl)butanimidamide hydrochloride (55), which underwent tandem intramolecular cyclization in an acidic medium to form 6,7-dihydro-5Hpyrrolo[ 1,2-a]imidazole (17a) (Scheme 21).37,38

Scheme 21
A convenient method for the synthesis of 6,7-dihydro-5H-pyrrolo[1,2-a]imidazol-3-amines 57a–e, promising substrates for heterocyclization processes, is the recyclization of 1,3-oxazole-4-carbonitriles 56a–e by the action of hydrazine hydrate (Scheme 22).39–41

Scheme 22
A one-pot version of the synthesis, which is promoted by a base and involves a cascade of addition and cyclization reactions of propargylamide 58 with trimethylsilyl cyanide, was successfully used to obtain 2,3-dihydro-1Hpyrrolo[ 1,2-a]imidazole-6-carbonitrile 59 (Scheme 23).42
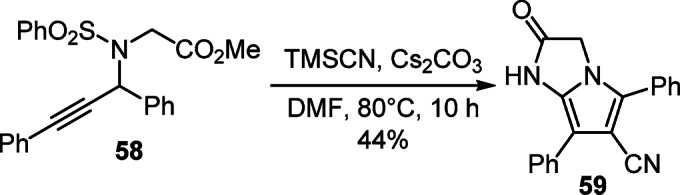
Scheme 23
THE SYNTHESIS METHODS OF TETRAHYDROPYRROLO[1,2-a]IMIDAZOLES
Annulation of the imidazole ring to the pyrrole ring
The indirect oxidation of 2-(pyrrolidin-1-yl)ethylamine (60) by monoamine oxidase (MAO-N-D5) in the presence of flavin adenine dinucleotide (FAD) was used to obtain 2,5,6,7-tetrahydro-3H-pyrrolo[1,2-a]imidazole (61) (no yield given, Scheme 24).43
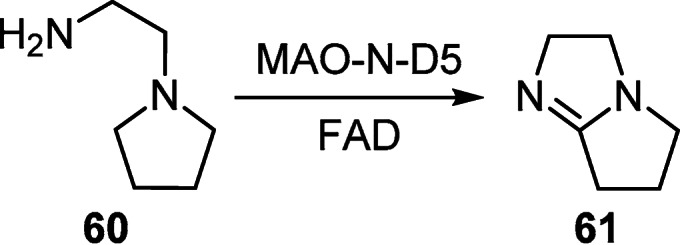
Scheme 24
Dehydrogenation of 1-phenyl-2-(2-phenylpyrrolidin-1-yl)-ethylamine (62a) with the help of the Hg(II)–EDTA system proved to be suitable for the formation of 2,5-diphenyl-2,5,6,7-tetrahydro-3Н-pyrrolo[1,2-a]imidazole (63) (Scheme 25).44 The introduction of chiral aminoethylpyrrolidine into this reaction made it possible to obtain an optically pure analog of compound 63 in 45% yield.
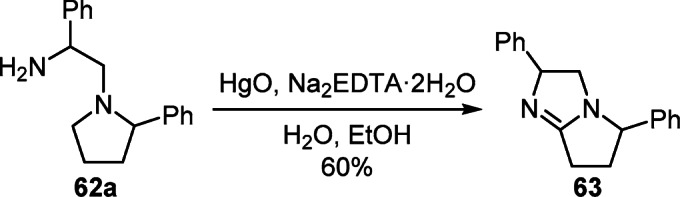
Scheme 25
Intramolecular aza-Wittig cyclization of ethyl 1-(2-azidoacetyl)-2-oxopyrrolidine-3-carboxylate 64 afforded 2,5,6,7-tetrahydro-3H-pyrrolo[1,2-а]imidazole 65 in 36% yield (Scheme 26).45

Scheme 26
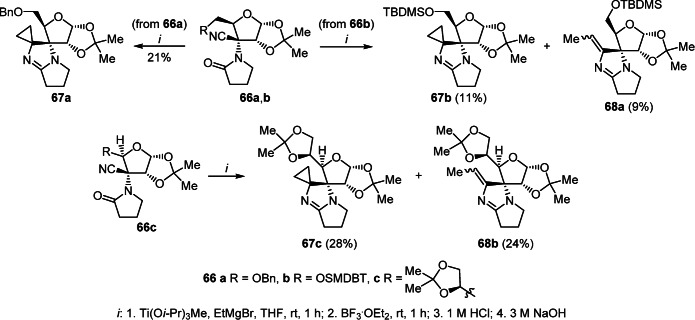
Cyclopropanation of the nitrile group of cyano-substituted furolactam 66а with the Ti(Oi-Pr)3Me–EtMgBr–BF3·OEt2 system was used to obtain spiro derivative of pyrrolo[1,2-а]imidazole 67а in 21% yield. A similar reaction of compounds 66b,c led to the formation of target products 67b,c and 2'-ethylidenepyrrolo[1,2-a]imidazole derivatives 68a,b as side products (Scheme 27).46
Scheme 27
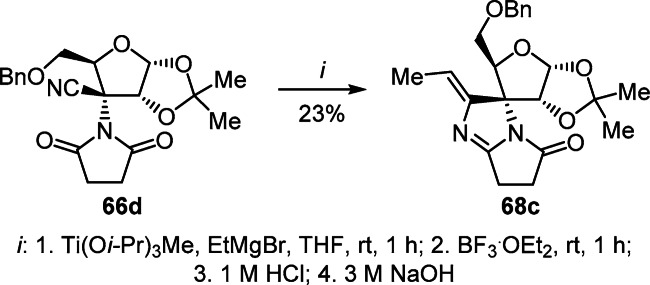
In turn, in a similar reaction of nitrile 66d containing a succinimide fragment, no cyclopropanation was observed; only annulation of the imidazole ring occurred leading to the isolation of 2'-ethylidenepyrrolo[1,2-a]imidazole 68c in low yield (Scheme 28).46
Scheme 28
The synthetic potential of pyrroliniminophosphazenes 69а–е as N,N-binucleophiles was successfully used in the reaction with oxalyl chloride to obtain methyl 2,3-dioxo- 2,3,5,6-tetrahydro-1H-pyrrolo[1,2-a]imidazole-7-carboxylates 70а–е (Scheme 29).47
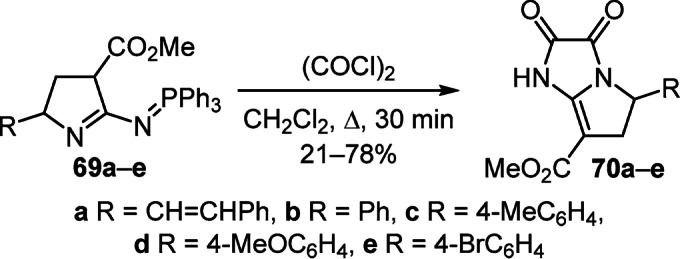
Scheme 29
Annulation of the pyrrole ring to the imidazole ring
A number of functionally substituted hydrogenated pyrrolo[1,2-а]imidazoles 73а–m were synthesized by a noncatalytic cascade reaction of heterocyclic ketenaminals 71а,с,е,f with N-substituted maleimides 72a–g in EtOH at room temperature48 or by heating under reflux in MeCN.49 Similar products 75а–f were obtained by cyclocondensation of ketenaminals 71а–d with arylpropiolates 74а–с (Scheme 30).50
Scheme 30
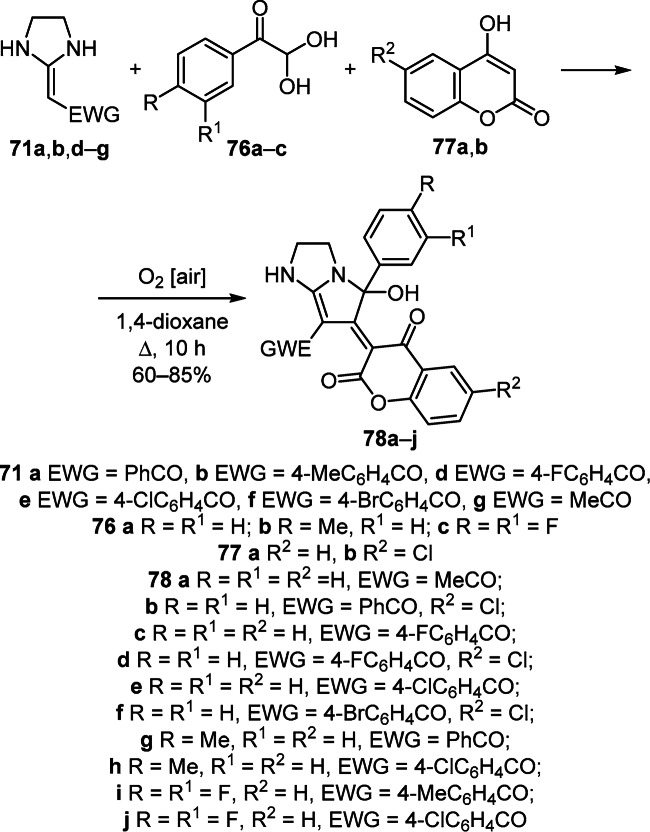
A three-component cascade reaction of aerobic oxidation implemented by the reaction of arylglyoxals 76a–c, 2-(imidazolidin-2-ylidene)-1-ethanones 71a,b,d–g, and 4-hydroxy-2H-chromen-2-ones 77a,b by heating under reflux in 1,4-dioxane was successfully employed for the synthesis of highly functionalized tetrahydropyrrolo[1,2-a]- imidazoles 78а–j (Scheme 31).51
Scheme 31
An attempt to synthesize tetrahydropyrrolo[1,2-a]- imidazol-5-one 81 by the [2+2+1] cycloaddition reaction of hexylacetylene (79), dihydroimidazole 80, and CO under radical initiation conditions was not entirely successful, because it led to the target product with a yield of only 3% (Scheme 32).52
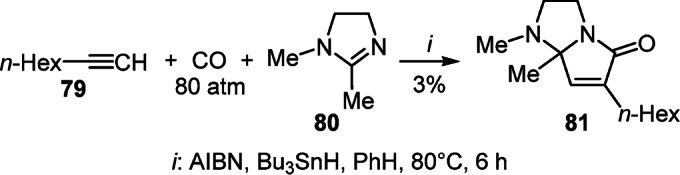
Scheme 32
A three-component reaction of dihydroimidazole 82a, dimethyl acetylenedicarboxylate 29b, and ethyl diazoacetate (83) catalyzed by organic copper(II) salts was used for the diastereoselective construction of the tricarboxylatesubstituted pyrrolo[1,2-а]imidazole 84. However, the yield of the target product did not exceed 14% (Scheme 33).53
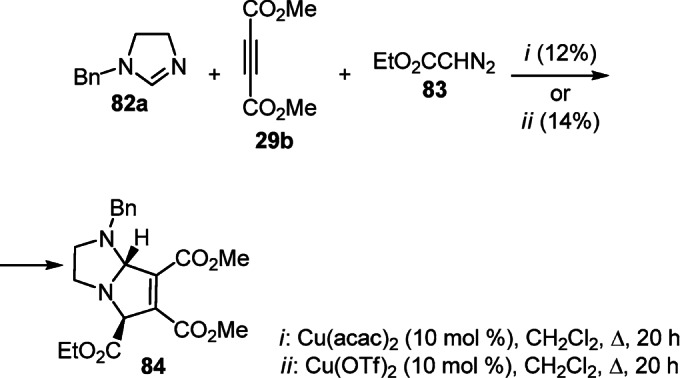
Scheme 33
A one-pot three-component reaction of diamines 85а,b and nitroketene dithioacetal (86) in the presence of diaroylacetylenes 87а,b led to the formation of 2,3,5,6- tetrahydro-1Н-pyrrolo[1,2-а]imidazoles 88а–d.54 A similar reaction with the participation of dialkyl acetylenedicarboxylates 29а,b afforded derivatives 89а–d (Scheme 34).55
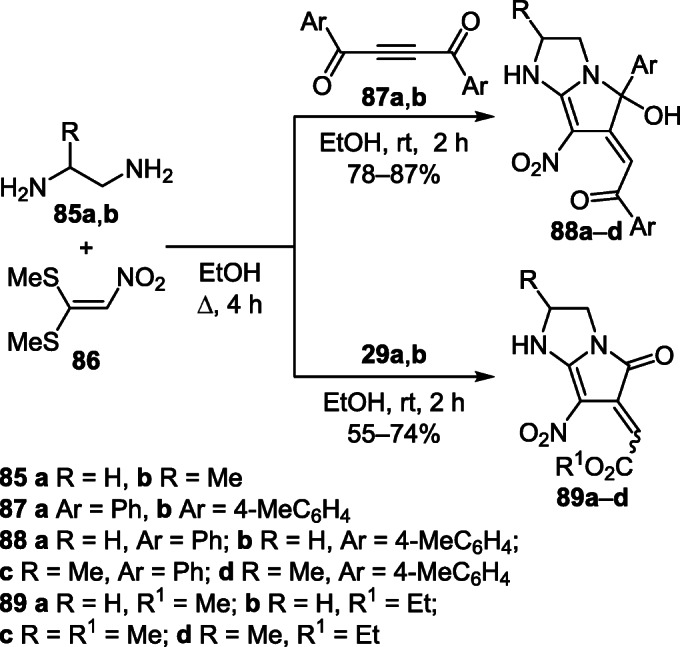
Scheme 34
A convenient two-step method for the synthesis of spiro-[[2]benzofuran-1,5'-pyrrolo[1,2-a]imidazole]diones 92а–j was carried out by the oxidation of cis-indenopyrrolo-[1,2-a]imidazoles 90а–j by NaIO4 to (pyrrolo[1,2-a]imidazol-5-yl)benzoic acids 91а–j, which underwent subsequent intramolecular cyclocondensation catalyzed by p-toluenesulfonic acid to give target diones 92а–j (Scheme 35).56
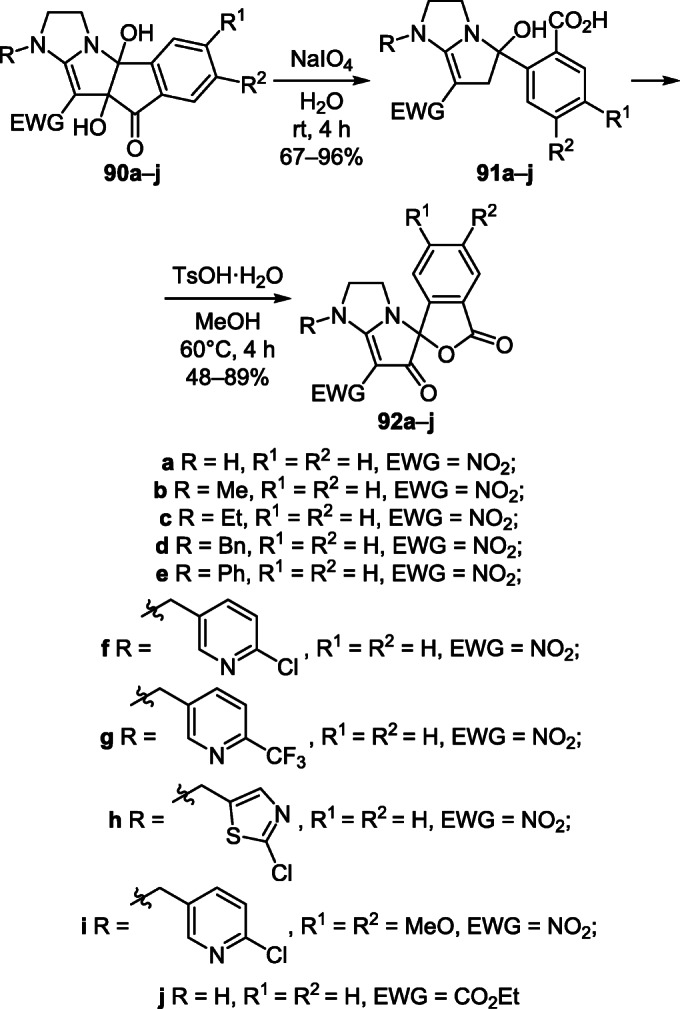
Scheme 35
Isomers of compounds 92, namely spiro[[2]benzofuran-1,6'-pyrrolo[1,2-a]imidazole]-3,5'(1'H)-diones 94а–k, were obtained via Pb(OAc)4-promoted oxidation of cisindeno[1',2':4,5]pyrrolo[1,2-a]imidazol-5(1H)-ones 93а–k (Scheme 36).56

Scheme 36
A method of radical borylation–cyclization of N-allylcyanamide 95 was developed for the construction of boroncontaining compound tetrahydropyrrolo[1,2-a]imidazole 97. The reaction is initiated by chemo- and regioselective addition of the NHC-boryl radical generated from zwitterion 96 to the aryl-substituted alkene fragment of compound 95, followed by cyclization at the cyanamide group (Scheme 37).57
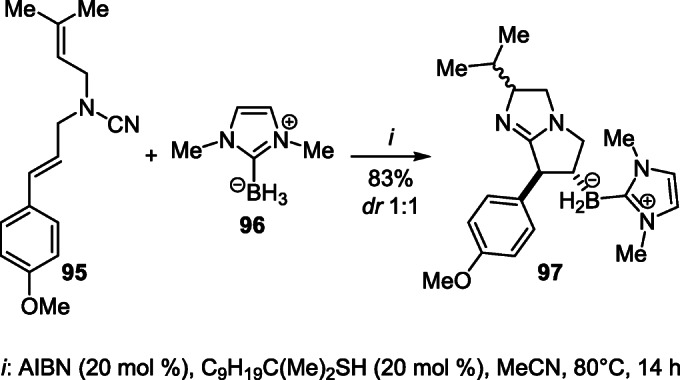
Scheme 37
Other methods
Oxidative rearrangement of (spiro)cyclobutane aminals proved an effective tool for constructing the pyrrolo[1,2-a]- imidazole framework. Thus, derivatives of (spiro)pyrrolo- [1,2-a]imidazoles 100a–d were formed as a result of the condensation of cyclobutanones 98a–d with (1S,2S)-1,2-diphenylethane-1,2-diamine (99) followed by oxidative rearrangement by the action of NBS (Scheme 38).58

Scheme 38
THE SYNTHESIS METHODS OF PERHYDROPYRROLO[1,2-a]IMIDAZOLES
Annulation of the imidazole ring to the pyrrole ring
The redox annulation reaction of pyrrolidines and α-ketoamides proved to be effective for the creation of combinatorial libraries of perhydrogenated pyrrolo[1,2-а]imidazoles 102a–z. It was found that N-alkyl-α-ketoamides 101а–с reacted with pyrrolidine 28a upon heating under reflux in PhMe in the presence of catalytic amounts of AcOH; in contrast, their N-aryl and N-hetaryl analogs 101d–у reacted with pyrrolidine 28a in the absence of acid. In the case of 2-phenylpyrrolidine 28b, pyrrolo[1,2-а]imidazole 102z was obtained by heating under reflux in xylene with the addition of 20 mol % AcOH (Scheme 39).59
Scheme 39
Benzoic acid was used as a catalyst in the synthesis of 1,3-diphenyltetrahydro-1H-pyrrolo[1,2-a]imidazol-2(3H)- one 102d, which made it possible to significantly reduce the reaction duration and obtain the target product in almost quantitative yield (Scheme 40).60 A new two-step one-pot approach based on the reaction of phenylglyoxylic acid (103), phenylisocyanate, and pyrrolidine 28a was also developed for the synthesis of this compound (Scheme 40).61
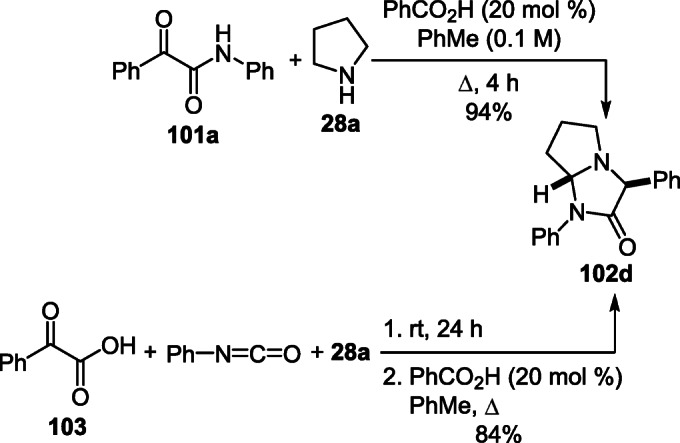
Scheme 40
L-Proline (32а) was also investigated in the cyclization reaction with decarboxylation with various α-ketoamides. It reacted smoothly and diastereoselectively with N-alkyl- (aryl,hetaryl)-α-ketoamides 101a–y, 104a–e to form the corresponding tetrahydro-1H-pyrrolo[1,2-a]imidazol-2(3H)- ones 102a–y, 105a–d in the form of the trans-isomers. By using 2-oxo-N-(p-tolyl)propanamide 104e, the target product 105e was isolated as a mixture of diastereomers in a 10:1 ratio in 56% yield (Scheme 41).62

Scheme 41
α-Silyloxyacrylamides 106a,b can also undergo a similar transformation. In a reaction with L-proline (32a) in i-PrOH under reflux, they are stereoselectively converted to tetrahydro-1H-pyrrolo[1,2-a]imidazol-2-ones 107а,b (Scheme 42).63
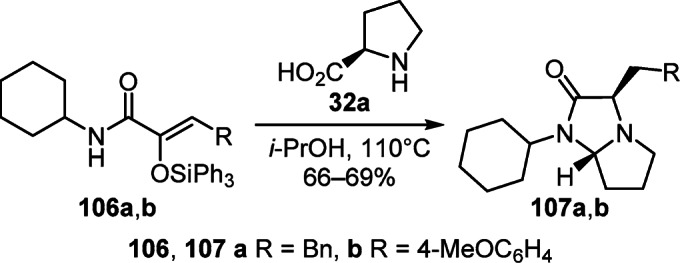
Scheme 42
A new biocatalytic route for the synthesis of tetrahydro-1H-pyrrolo[1,2-a]imidazol(e)-2(3H)-(thi)ones 109а–g was proposed, which involves the enzymatic intramolecular C–H amination of N-substituted 2-(pyrrolidin-1-yl)acetamides 108а–g by the action of mutant cytochrome P450BM3 (CYP102A1) enzymes and led to target products in 55–97% yields (Scheme 43).64
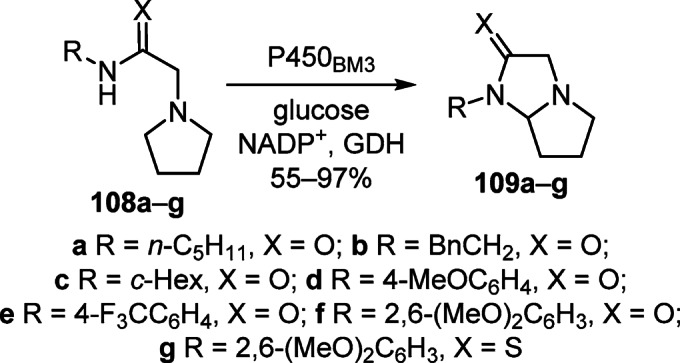
Scheme 43
These studies demonstrated the tolerance of the P450BM3 mutants to functional groups in both the amide and pyrrolidine fragments of the substrates, as well as the high chemoselectivity of the amination of the C–H bond. In particular, methyl L-prolinate derivative 108h underwent cyclization exclusively to product 109h in 98% yield (Scheme 44).64

Scheme 44
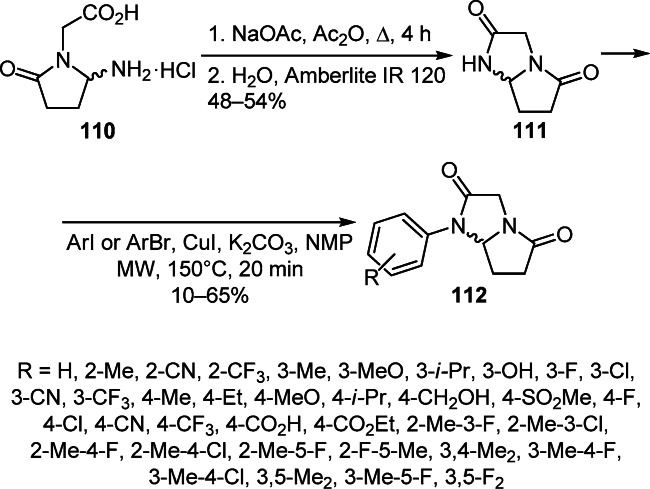
The intramolecular cyclization of 2-(2-amino-5-oxopyrrolidin-1-yl)acetic acid hydrochloride (110) leading to dimiracetam (111) presents an efficient method for the annulation of the perhydropyrrolo[1,2-a]imidazole ring. Its modification gave 1-arylpyrrolo[1,2-a]imidazolediones 112, which possess an antihyperalgesic effect against neuropathic pain induced by both chronic constriction injury of the sciatic nerve and streptozotocin (Scheme 45).65
Scheme 45
The nitrile group of polysubstituted succinimide 113 was used for its one-step conversion to perhydropyrrolo[1,2-a]-imidazole 114 (Scheme 46).66

Scheme 46
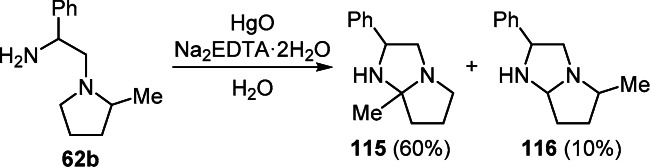
Intramolecular cyclization of 1-aminoethylpyrrolidine 62b by the action of HgO in the presence of ethylenediaminetetraacetic acid disodium salt (Na2EDTA·2H2O) led to the preferential formation of pyrrolo[1,2-a]imidazole 115 by dehydrogenation of the С–Н bond in position 2 of the pyrrolidine ring. The isomeric product 116 is the result of dehydrogenation at position 5 of the ring (Scheme 47).44
Scheme 47
A simple approach to the synthesis of fully hydrogenated pyrrolo[1,2-a]imidazolones was developed based on the intramolecular amination of the C–H bond of pyrrolidine by the action of a simple catalytic system consisting of FeCl2 and β-diketiminate L1 as a ligand. It was shown that heating α-azidoacylpyrrolidine 117 in MeCN with the addition of Boc2O to activate cyclization leads to 3-oxohexahydro-1H-pyrrolo[1,2-a]imidazole 118 in 33% yield.67 Using the Fe(II)–1,2-bis(diphenylphosphino) benzene ([Fe(dppbz)]Cl2) as a catalyst in the absence of a ligand made it possible to increase the yield of the target product to 92% (Scheme 48).

Scheme 48
Annulation of the pyrrole ring to the imidazole ring
The 1,3-dipolar cycloaddition reaction of 4,5-dihydroimidazolium ylides D obtained by the reaction of dihydroimidazole 82а,b with ethyl 2-diazoacetate 83 with fumaric acid esters 119а,b did not show high stereoselectivity and led to a diastereomeric mixture of derivatives 120а–d and 121а,b. In the case of fumarodinitrile 122, pyrrolo[1,2-a]imidazole 123 was isolated in low yield (Scheme 49).53
Scheme 49
The three-component reaction of dihydroimidazoles 82а,с, esters or nitrile of bromoacetic acid 125а–d, and activated ethylenes 126а,b was successfully used to annulate the pyrrole ring and obtain functionally substituted perhydropyrrolo[1,2-а]imidazoles 127a–l (Scheme 50).69 Similar reaction conditions proved to be suitable for the synthesis of (3R)-phenylpyrrolo[1,2-a]-imidazoles 127m–x using chiral dihydroimidazoles 124а–с, bromoacetic acid esters 125а–с, and vinyl sulfones 126b,с.69
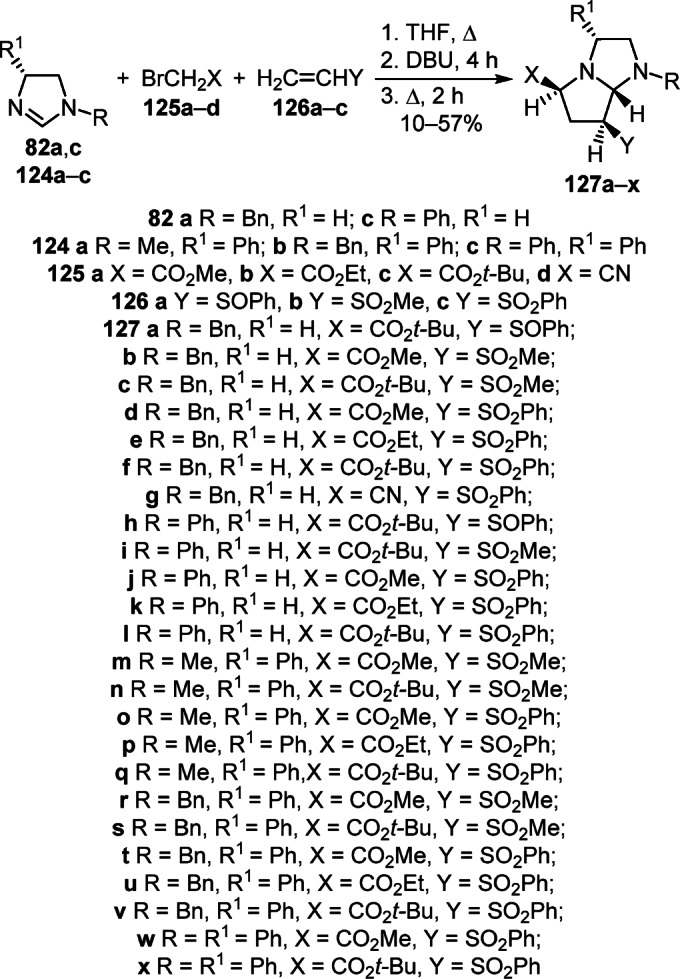
Scheme 50
Upon expanding the number of activated alkenes as dipolarophiles, interesting results were obtained in the case of methacrylic acid derivatives. Thus, the reaction of (3S)-phenyldihydroimidazole 128, bromoacetic acid esters 125а,с, and methyl methacrylate (126d) resulted in diastereoselective formation of solely endo-addition products 129a,c. Nitriles 129b,d were isolated together with a small amount of compounds 130а,b, respectively (endo:exo = 8:1) in a similar transformation involving methacrylonitrile (126е) (Scheme 51).
Scheme 51
The reaction of enantiomeric (3R)-phenyldihydroimidazole 124b, bromoacetic acid esters 125а,с, and methyl methacrylate (126d) also proceeded diastereoselectively with the formation of endo-addition products 129е,g. When using methacrylonitrile (126е), the target nitrile 129f was obtained in admixture with a small amount of product 130с (endo:exo = 11:1), while adduct 129h was produced in admixture with a small amount of exo-addition product 130d (endo:exo = 8:1, Scheme 51).70
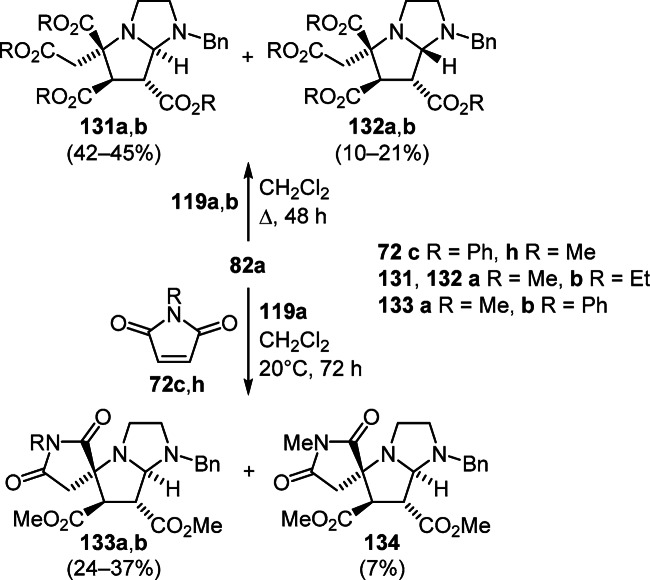
A method for the synthesis of hexahydropyrrolo[1,2-a]- imidazoles was developed in which 4,5-dihydroimidazolium ylides were obtained by conjugated addition with proton transfer from dihydroimidazoles and difunctionalized electron-deficient alkenes. Thus, stereoisomeric products 131 and 132 were synthesized by the reaction of 1-benzyl-4,5-dihydro-1H-imidazole (82а) with fumaric acid esters 119а,b. Modification of this reaction by the addition of N-methylmaleimide 72h was successfully used for the synthesis of diastereomeric spiro[pyrrolidine-3,5'-pyrrolo[1,2-a]imidazoles] 133a and 134. It is noteworthy that in the case of N-phenylmaleimide 72с the conversion proceeded diastereoselectively and led to the formation of solely product 133b (Scheme 52).71
Scheme 52
Imidazolium salts 135a,b when treated with 1 equiv of n-BuLi were prone to cyclization to pyrrolo[1,2-a]-imidazole spiro derivatives 136a,b, the reaction of which with synergistic mixtures of n-BuLi or PhLi and LiN(SiMe3)2 was used to synthesize saturated complexes of N-heterocyclic carbenes, in particular, homobimetallic complexes of lithium, sodium, and potassium (Scheme 53).72,73
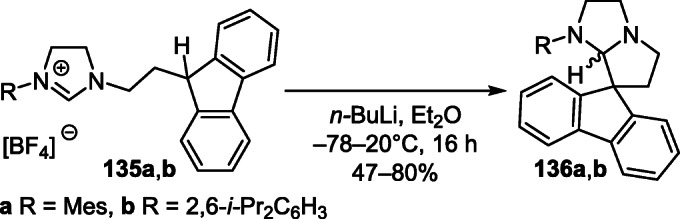
Scheme 53
The catalytic redox reaction of N-alkenyl-substituted imidazolium salts 137a–e with the participation of the Ni(0)/Ni(II) redox system proved to be effective for the mild synthesis of pyrrolo[1,2-a]imidazolium salts 138a–e (Scheme 54).74
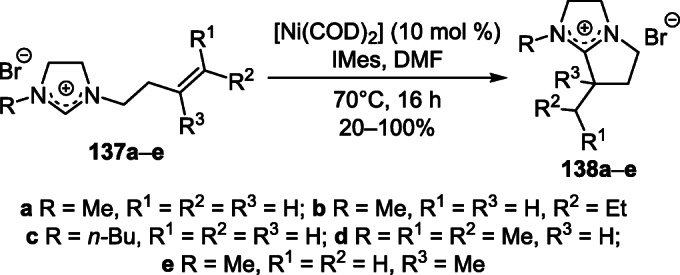
Scheme 54
Other methods
Principally, other synthesis methods include transformations in which the target perhydrogenated bicyclic system is formed from two, as a rule, acyclic reagents. A good example of this approach is the cyclocondensation of ethylenediamines 85а,b with ethyl levulinate (139) in EtOH leading to the formation of pyrrolo[1,2-а]imidazoles 140а,b.75–77 In turn, the reaction of ethylenediamine 85а with methyl 1-(2-oxoethyl)cyclohexanecarboxylate (141) gave pyrrolo[1,2-a]imidazole spiro derivative 142 (Scheme 55).78

Scheme 55
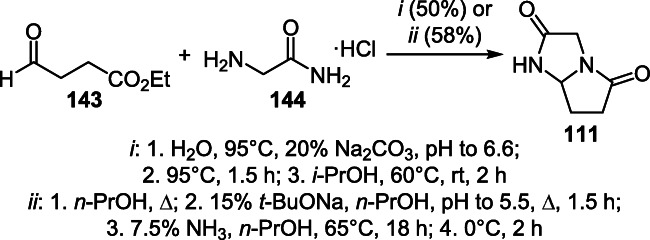
Condensation of ethyl 4-oxobutanoate (143) with glycinamide hydrochloride (144) forms the basis of a one-pot industrial method for the preparation of tetrahydro-5H-pyrrolo[1,2-а]imidazole-2,5(3H)-dione (dimiracetam) (111) (Scheme 56).79,80
Scheme 56
Cyclocondensation of L-α-aminohydroxamic acids 145a–e with 4-oxopentanoic acid (146) presents a convenient method for the synthesis of 1-hydroxytetrahydro-5Hpyrrolo[1,2-а]imidazole-2,5(3H)-diones 147a–e. Notably, in the case of acids 145а–с, it is expedient to carry out the reaction by heating in PhMe, whereas for their aromatic analogs 145d,е, successive heating in i-РОН and PhMe turned out to be optimal leading to target products 147d,e in almost quantitative yields (Scheme 57).81

Scheme 57
N-Substituted amides of alanine 148а or valine 148b were patented as effective substrates in reductive cyclization, which leads to the formation of 3-oxopyrrolo[1,2-a]imidazoles 149а,b (no yields given), which are disposed to competitive inhibition of melanocortin receptors (Scheme 58).82

Scheme 58
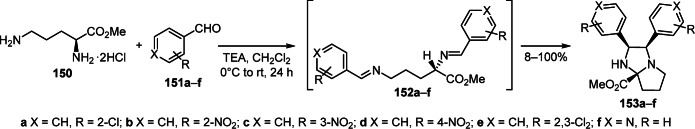
A novel approach to the diastereoselective synthesis of hexahydro-1H-pyrrolo[1,2-a]imidazoles 153a–f is based on the reaction of L-ornithine methyl ester dihydrochloride (150) with aromatic aldehydes 151а–f and is realized via the formation of bis-Schiff bases 152а–f followed by intramolecular 1,3-dipolar cycloaddition (Scheme 59).83
Scheme 59
Cyclocondensation of L-α-amino acid phenylhydrazides 154a–f with 2,3-O-isopropylidene-L-erythruronolactone (155) in the presence of a catalytic amount of p-toluenesulfonic acid (p-TSA) afforded diastereomerically pure 3-substituted 6,7-[propane-2,2-diylbis(oxy)]-1-(phenylamino)-tetrahydro-5H-pyrrolo[1,2-a]imidazole-2,5(3H)-diones 156a–f, acid hydrolysis of which gave their 6,7-dihydroxy analogs 157a–f (Scheme 60).84

Scheme 60
The product of condensation of N-Boc-L-alanine (159) with 3-(1,3-dioxolan-2-yl)propanamine (158), in situ generated amide F, upon treatment with SnCl2·2H2O was successively subjected to deacetalization/bicyclization processes, which resulted in the formation of a diastereomeric mixture of 3-oxopyrrolo[1,2-a]imidazole 160 (Scheme 61).85
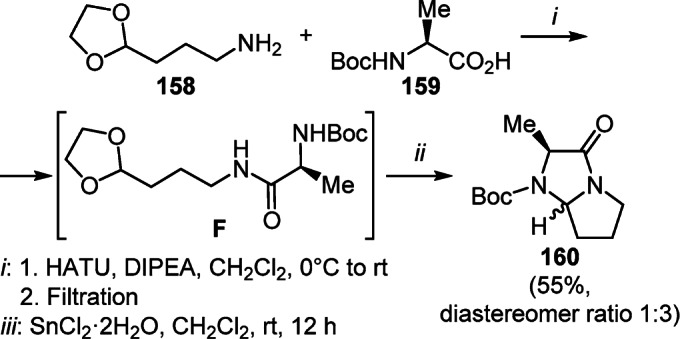
Scheme 61
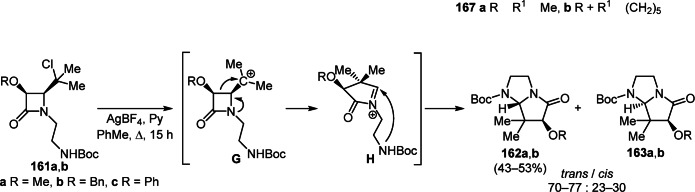
An interesting example of the synthesis of diastereomers of 5-oxohexahydro-1H-pyrrolo[1,2-a]imidazoles 162, 163 a,b involves ring expansion of monocyclic β-lactams. For example, upon treatment with AgBF4 and pyridine in PhMe, cis-azetidin-2-ones 161а,b were diastereoselectively converted to the target products via rearrangement of carbocation G and intramolecular cyclization of N-acyliminium intermediates H (Scheme 62).86
Scheme 62
An original approach to the preparation of 5-methylidenehexahydropyrrolo[1,2-a]imidazoles 166a,b is based on the reaction of alkynylcyclopropanes 164a,b with lithium 2-aminoethylamide generated from ethylenediamine 85a and n-BuLi. The reaction proceeds via the intermediate formation of conjugated alkynylcyclopropene I with the characteristic combination of two highly reactive functionalities, a triple bond, and an unsaturated threemembered ring. Its subsequent hydrolysis in the case of a bulky tert-butyl substituent proceeds regioselectively with the formation of the target product 165 exclusively as the E-isomer. At the same time, its 5-benzylidene analogs 166а,b were isolated as a mixture of Е- and Z-isomers in ratios of 4:1 and 4.2:1, respectively (Scheme 63).87,88

Scheme 63
The synthesis of 5-benzylidenepyrrolo[1,2-a]imidazoles 166a,b as a mixture of E- and Z-isomers in the ratio of 5:1 and 4.5:1, respectively, was also carried out by cyclization of acetylenaldehydes 167a,b with ethylenediamine 72a in the superbasic DMSO–KOH medium (Scheme 64).89.90
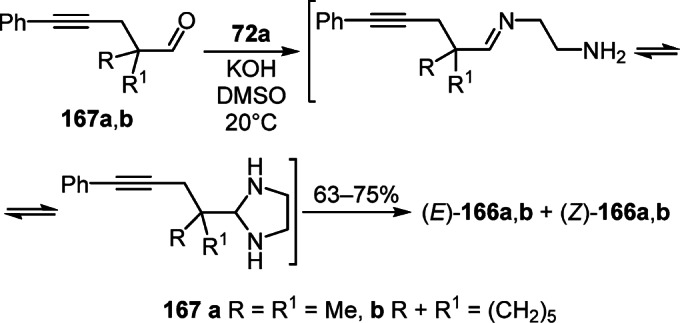
Scheme 64
ASPECTS OF APPLICATION OF PYRROLO[1,2-а]IMIDAZOLES
The use of pyrrolo[1,2-a]imidazoles as organocatalysts
Pyrrolo[1,2-a]imidazole derivatives have found wide application in various fields of organic synthesis as selective organocatalysts. Thus, 7-alkoxy-substituted chiral 6,7-dihydro-5H-pyrrolo[1,2-a]imidazoles 168а–d were successfully used in the asymmetric Steglich rearrangement.26 In addition, derivatives 168а–c were first used for the catalytic asymmetric synthesis of chiral compounds (Fig. 3).91
Figure 3.
The catalysts for asymmetric processes 168a–d and stereoselective phosphoramidation 169, as well as catalysts for the synthesis of remdesivir 170a,b.
An original low molecular weight catalyst 169 was constructed based on the scaffold of a chiral 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole (Fig. 3). It promotes the insertion of P-stereogenic phosphoramidates into nucleoside structures via a dynamic stereoselective process.92
More recently, reports have appeared on the use of (pyrrolo[1,2-a]imidazol-7-yl)carbamates 170а,b for organocatalytic asymmetric phosphoramidation of one of the intermediates in the synthesis of the drug remdesivir which is used to treat COVID-19 (Fig. 3).93,94
Chiral esters based on pyrrolo[1,2-a]imidazoles 171а–е proved to be effective catalysts for the enantioselective Black rearrangement (Fig. 4).28 In addition, ((R)-6,7- dihydro-5H-pyrrolo[1,2-a]imidazol-7-yl)acetate (171a) has found application in direct catalytic enantioselective C-acylation for the construction of the quaternary stereocenter of 3-substituted benzofuran-2(3H)-ones.95
Figure 4.
The catalysts of enantioselective processes 171a–e, kinetic separation of aryl and hetaryl alkyl carbinols 172, and polymerization initiators pyrrolo[1,2-а]imidazoles 173a,b.
(S)-7-Cyclohexyl-3-(pyrrolidin-1-yl)-6,7-dihydro-5Hpyrrolo[ 1,2-a]imidazole (172) was successfully used for the kinetic separation of aryl and hetaryl alkyl carbinols with high enantioselectivity (Fig. 4).96
Chiral dialkylaluminum complexes of 6,7-dihydro-5Hpyrrolo[1,2-а]imidazol-7-ols 173а,b were tested as effective initiators of ring opening of ε-caprolactone during polymerization (Fig. 4).97
The use of pyrrolo[1,2-a]imidazoles as ionic liquids
Another area of practical application of functionalized pyrrolo[1,2-a]imidazoles is ionic liquids. Affinity ionic liquids of pyrrolo[1,2-a]imidazolium 174a,b are capable of binding to peptides and proteins, which has been used for liquid-liquid extraction and purification of hexahistidinetagged (His-tagged) proteins (Fig. 5).37,98
Figure 5.
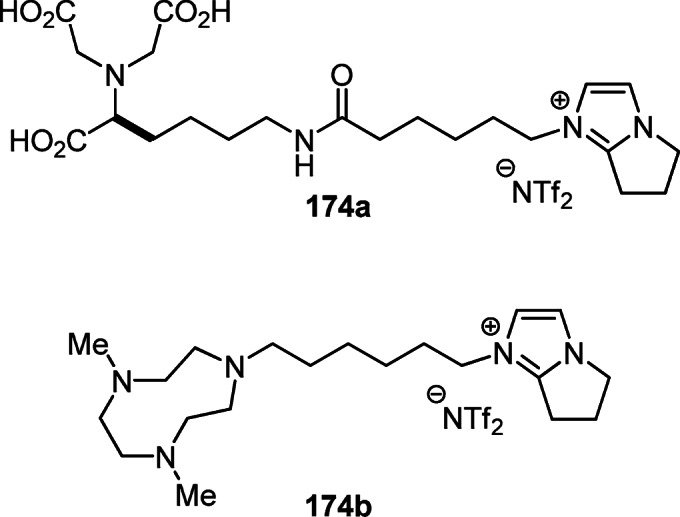
The affinity ionic liquids 174a,b capable of binding to biomacromolecules.
Quartz crystal microbalance (QCM) chips thinly coated with sensitive ionic liquids 175а–d proved effective for the chemoselective detection of aldehydes, ketones, and amines in gases.99,100 The use of 1-butylpyrrolo[1,2-a]-imidazolium (trifluoromethanesulfonyl)imide (175c) for the separation of Th-227 and Ac-225 isotopes by extraction with N,N,N',N'-tetraoctyldiglycolamide also deserves attention (Fig. 6).38
Figure 6.
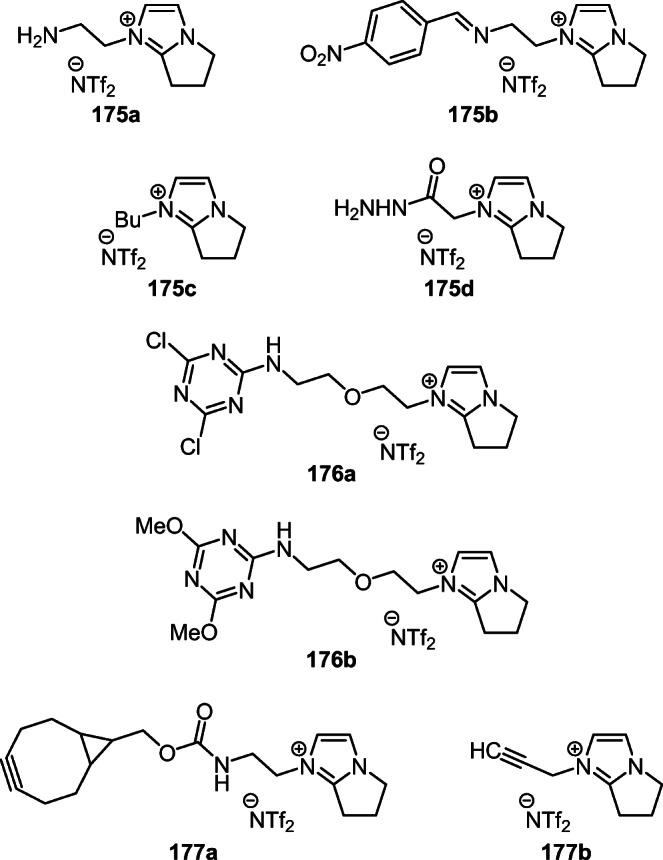
Ionic liquids 175a–d (for effective gas detection and isotope separation), 176a,b (for the determination of gaseous amines and alcohols), and 177a,b (for the determination of organic azides).
In turn, the 1,3,5-triazine-containing ionic liquids 176a,b on QCM chips seem to be very promising for chemoselective detection of gaseous amines and alcohols (Fig. 6).101
Equally important is the development of the specialized ionic liquids 177a,b on QCM chips for on-line and chemoselective real-time detection of organic azides (Fig. 6).102
Biomedical potential of pyrrolo[1,2-a]imidazoles
Heterocyclic compounds based on the pyrrolo[1,2-a]- imidazole ring are attractive objects for biomedical research. Thus, 3-(3,4-dichlorophenyl)-1-[(4-phenoxyphenylcarbamoyl) methyl]-6,7-dihydro-5H-pyrrolo[1,2-a]-imidazol-1-ium chloride (178) has a wide spectrum of antimicrobial activity against Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Cryptococcus neoformans. Notably, compound 178 is characterized by high hemolytic activity against human erythrocytes and cytotoxicity against the HEK-293 cell line, but has low in vivo toxicity in mice (LD50 >2000 mg/kg) (Fig. 7).15
Figure 7.
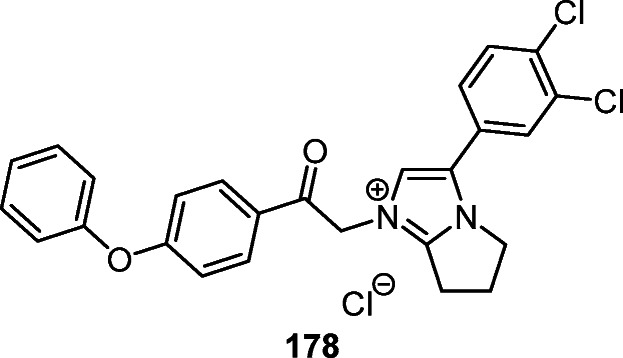
The quaternary salt of pyrrolo[1,2-a]imidazol-1-ium 178 with antimicrobial activity.
Effective inhibitors of the WIN region of the WDR5 scaffolding protein, which regulates chromatin and is overexpressed in various types of cancer, were identified among 2-aryl-6,7-dihydro-5H-pyrrolo[1,2-a]imidazoles. Derivatives 179а–с are characterized by dissociation constants <10 nM and micromolar cellular activity against acute myeloid leukemia (Fig. 8).12
Figure 8.
The selective inhibitors of the WIN region of the WDR5 scaffolding protein 179а–с.
Modified pyrrolo[1,2-a]imidazoles 180 were evaluated as degraders of the WDR5 protein, but despite only inhibiting a subset of WDR5 protein interactions, chemically induced proteasomal degradation of the WDR5 protein could represent an elegant way to target all oncogenic processes (Fig. 9).13
Figure 9.

The WDR5 protein degraders pyrrolo[1,2-а]imidazoles 180.
3-Aryl-substituted 6,7-dihydro-5H-pyrrolo[1,2-a]imidazoles 14d,f may find application in the treatment of castration-resistant prostate cancer. These molecules have been shown to block the nuclear localization of androgen receptors in prostate cancer (Fig. 10).9,103
Figure 10.

Pyrrolo[1,2-a]imidazoles 14d,f with anticancer activity.
(1S,3R)-3-Acetamido-N-[4-(6,7-dihydro-5H-pyrrolo[1,2-a]-imidazol-3-yl)pyridin-2-yl]cyclohexanecarboxamides 181а–с inhibit cyclin-dependent kinase CDK9 and may be useful important is the effect of compounds 181 on phosphorylation of the Ser2 residue of RNA polymerase II in the breast cancer cell line (MCF7) and on MV4-11 cells of biphenotypic B-myelomonocytic leukemia (Fig. 11).16
Figure 11.
The inhibitors of cyclin-dependent kinase CDK9 181а–с.
Structural modification of the pyrrolo[1,2-a]imidazole ring was used to construct derivative 182 containing a cereblon ligand that binds to E3 ubiquitin ligase, as well as a fragment capable of binding to the androgen receptor and inhibiting it (Fig. 12).17
Figure 12.
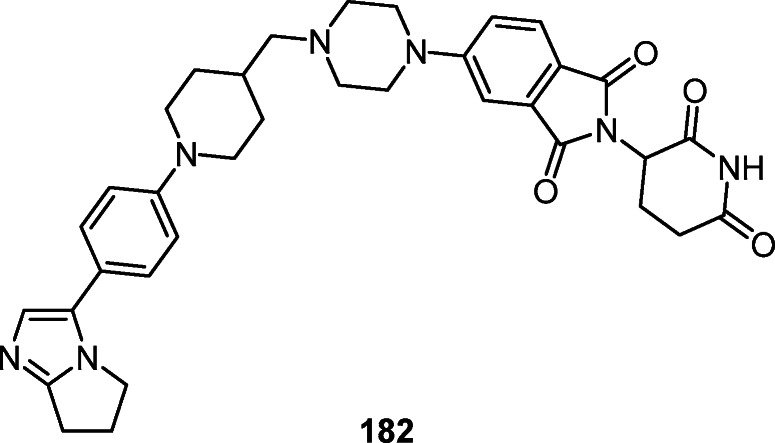
The androgen receptor inhibitor 182.
The optically pure pyrrolo[1,2-a]imidazoles 22, 183а,b are progesterone receptor (PR) antagonists and are useful in the treatment of endometriosis, uterine fibroids, and related conditions as well as in the treatment of breast, ovarian, or endometrial cancer (Fig. 13).18
Figure 13.

The progesterone receptor antagonists 22, 183а,b.
6,7-Dihydro-5H-pyrrolo[1,2-a]imidazole-2-sulfonamide 184 has the ability to inhibit the activation of the NLRP3 inflammasome and may be useful in the treatment of a wide range of diseases in which the NLRP3 inflammasome is considered the key inflammation-regulating factor.41
In turn, N-(pyrrolo[1,2-а]imidazol-3-yl)acetamide 185 was tested as an inhibitor of human tropomyosin receptor kinase A (hTrkA) (Fig. 14).104
Figure 14.

The biologically active pyrrolo[1,2-а]imidazoles 184–187.
Perhydropyrrolo[1,2-a]imidazole 186 was revealed as a selective antagonist of the melanocortin-4 receptor (pA2 = 8.086 M) and also weakens the binding of an agonist, including α-melanocyte-stimulating hormone, and of an inverse agonist, including a protein related to the melanocortin receptor (Fig. 14).105–108 1-(p-Tolyl)tetrahydro-5H-pyrrolo[1,2-a]imidazole-2,5(3H)-dione (187) may be promising for treatment and prevention of peripheral neurotoxicity induced by chemotherapy (Fig. 14).109,110
To conclude, the analysis of literature sources indicates that, in most cases, the construction of various pyrroloimidazole systems uses the annulation of the imidazole ring to the pyrrole ring or vice versa. However, ring expansion reactions, rearrangements, recyclizations, cascade and multicomponent reactions, as well as one-pot methods for the formation of the pyrroloimidazole framework are of considerable interest among the methods of synthesis of pyrrolo[1,2-a]imidazoles. The practical significance of partially and fully hydrogenated pyrrolo[1,2-a]imidazoles is due to their use in organic synthesis as selective catalysts, ionic liquids in analytical sensors, and for the creation of pharmacologically active substances.
Footnotes
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(12), 661–680
Contributor Information
Lesya M. Saliyeva, Email: saliieva.lesia@vnu.edu.ua
Irina V. Dyachenko, Email: irina.chemii@gmail.com
Mykhailo V. Vovk, Email: mvovk@ioch.kiev.ua
References
- 1.Pinza M, Farina C, Cerri A, Pfeiffer U, Riccaboni MT, Banfi S, Biagetti R, Pozzi O, Magnani M, Dorigotti L. J. Med. Chem. 1993;36:4214. doi: 10.1021/jm00078a011. [DOI] [PubMed] [Google Scholar]
- 2.Roberts LR, Fish PV, Storer RI, Whitlock GA. Bioorg. Med. Chem. Lett. 2009;19:3113. doi: 10.1016/j.bmcl.2009.03.166. [DOI] [PubMed] [Google Scholar]
- 3.Kochergin PM, Druzhinina AA, Palei RM. Chem. Heterocycl. Compd. 1966;2:108. doi: 10.1007/BF00955613. [DOI] [Google Scholar]
- 4.De Benneville, P. L.; Niederhauwer, W. D. US Patent 3267082.
- 5.Chimirri A, Grasso S, Monforte AM, Monforte P, Zappala M. Farmaco. 1995;50:401. [PubMed] [Google Scholar]
- 6.Suffert, J. In Comprehensive Heterocyclic Chemistry III; Cossy, J., Ed.; 2008, Vol. 11, p. 41.
- 7.Suffert J. In Comprehensive Heterocyclic Chemistry IV. 2022;11:38. [Google Scholar]
- 8.Grinev VS, Egorova AY. Chem. Heterocycl. Compd. 2016;52:785. doi: 10.1007/s10593-016-1966-8. [DOI] [Google Scholar]
- 9.Wang, Z.; Nelson, J. B.; Nguyen, M. M. B.; Lazo, J. S.; Johnston, P. A.; Wipf, P. WO Patent 2013055793.
- 10.Stevens, M. Y.; Rozycka-Sokolowska, E.; Andersson, G.; Odell, L. R.; Marciniak, B.; Joule, J. A. ARKIVOC2015, (v), 219.
- 11.Shvydenko T, Nazarenko K, Shvydenko K, Boron S, Gutov O, Tolmachev A, Kostyuk A. Tetrahedron. 2017;73:6942. doi: 10.1016/j.tet.2017.10.053. [DOI] [Google Scholar]
- 12.Wang F, Jeon KO, Salovich JM, Macdonald JD, Alvarado J, Gogliotti RD, Phan J, Olejniczak ET, Sun Q, Wang S, Camper D, Yuh JP, Shaw JG, Sai J, Rossanese OW, Tansey WP, Stauffer SR, Fesik SW. J. Med. Chem. 2018;61:5623. doi: 10.1021/acs.jmedchem.8b00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dölle A, Adhikari B, Krämer A, Weckesser J, Berner N, Berger L-M, Diebold M, Szewczyk M, Barsyte- Lovejoy D, Arrowsmith C, Gebel J, Löhr F, Dötsch V, Eilers M, Heinzlmeir S, Kuster B, Sotriffer C, Wolf E, Knapp S. J. Med. Chem. 2021;64:10682. doi: 10.1021/acs.jmedchem.1c00146. [DOI] [PubMed] [Google Scholar]
- 14.Shvydenko T, Nazarenko K, Shvydenko K, Rusanov E, Tolmachev A, Kostyuk A. ChemistrySelect. 2019;4:8450. doi: 10.1002/slct.201901648. [DOI] [Google Scholar]
- 15.Demchenko S, Lesyk R, Yadlovskyi O, Zuegg J, Elliott AG, Drapak I, Fedchenkova Y, Suvorova Z, Demchenko A. Molecules. 2021;26:4253. doi: 10.3390/molecules26144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlaam, B.; De Savi, C.; Hawkins, J.; Hird, A.; Lamb, M.; Pike, K.; Vasbinder, M. US Patent 20160376287.
- 17.Crew, A. P.; Hornberger, K. R.; Snyder, L. B.; Zimmermann, K.; Wang, J.; Berlin, M.; Crews, C. M.; Dong, H. US Patent 20180099940.
- 18.Gibson, K. R.; Green, M. P.; Underwood, T. J.; Wakenhut, F. WO Patent 2010032200.
- 19.Estrada, A. A.; Feng, J. A.; Lyssikatos, J. P.; Sweeney, Z. K.; De Vicente Fidalgo, J. WO Patent 2017087905.
- 20.Whitlock GA, Brennan PE, Roberts LR, Stobie A. Bioorg. Med. Chem. Lett. 2009;19:3118. doi: 10.1016/j.bmcl.2009.03.162. [DOI] [PubMed] [Google Scholar]
- 21.Holladay, M. W.; Liu, G. WO Patent 2017019804.
- 22.Arepally S, Babu VN, Bakthadoss M, Sharada DS. Org. Lett. 2017;19:5014. doi: 10.1021/acs.orglett.7b01840. [DOI] [PubMed] [Google Scholar]
- 23.Maity S, Pathak S, Pramanik A. Tetrahedron Lett. 2013;54:2528. doi: 10.1016/j.tetlet.2013.03.017. [DOI] [Google Scholar]
- 24.Reddy CN, Sathish M, Adhikary S, Nanubolu JB, Alarifi A, Maurya RA, Kamal A. Org. Biomol. Chem. 2017;15:2730. doi: 10.1039/C7OB00299H. [DOI] [PubMed] [Google Scholar]
- 25.Adiyala PR, Jang S, Vishwakarma NK, Hwang Y-H, Kim D-P. Green Chem. 2020;22:1565. doi: 10.1039/C9GC03496J. [DOI] [Google Scholar]
- 26.Zhang Z, Xie F, Jia J, Zhang W. J. Am. Chem. Soc. 2010;132:15939. doi: 10.1021/ja109069k. [DOI] [PubMed] [Google Scholar]
- 27.Gomes, J. C.; Rodrigues, M. T., Jr.; Moyano, A.; Coelho, F. Eur. J. Org. Chem.2012, 6861.
- 28.Wang M, Zhang Z, Liu S, Xie F, Zhang W. Chem. Commun. 2014;50:1227. doi: 10.1039/C3CC47455K. [DOI] [PubMed] [Google Scholar]
- 29.Patel, S.; Hamilton, G.; Zhao, G.; Chen, H.; Daniels, B.; Stivala, C. WO Patent 2019012063.
- 30.De Miranda, A. S.; Gomes, J. C.; Rodrigues, M. T.; Costa, I. C. R.; Almeida, W. P.; Lopes, R. de O.; Miranda, L. S. M.; Coelho, F.; de Souza, R. O. M. A. J. Mol. Catal. B: Enzym.2013, 91, 77.
- 31.Wang Y-X, Qi S-L, Luan Y-X, Han X-W, Wang S, Chen H, Ye M. J. Am. Chem. Soc. 2018;140:5360. doi: 10.1021/jacs.8b02547. [DOI] [PubMed] [Google Scholar]
- 32.Joshi MS, Lansakara AI, Pigge FC. Tetrahedron Lett. 2015;56:3204. doi: 10.1016/j.tetlet.2014.12.074. [DOI] [Google Scholar]
- 33.Hou L, Wang Y, Tong X. Tetrahedron Lett. 1804;2018:59. [Google Scholar]
- 34.Popescu, M. V.; Mekereeya, A.; Alegre-Requena, J. V.; Paton, R. S.; Smith, M. D. Angew. Chem., Int. Ed.2020, 59, 23020. [DOI] [PMC free article] [PubMed]
- 35.Walker JA, Stanley LM. Org. Biomol. Chem. 2016;14:9981. doi: 10.1039/C6OB01956K. [DOI] [PubMed] [Google Scholar]
- 36.Li K, Huang R, Chen L, Lv Y, Yan S-J. Org. Lett. 2020;22:8210. doi: 10.1021/acs.orglett.0c02689. [DOI] [PubMed] [Google Scholar]
- 37.Tseng, M.-C.; Tseng, M.-J.; Chu, Y.-H. Chem. Commun.2009, 7503.
- 38.Luo HM, Boll RA, Bell JR, Dai S. Radiochim. Acta. 2012;100:771. doi: 10.1524/ract.2012.1950. [DOI] [Google Scholar]
- 39.Chumachenko SA, Shablykin OV, Vasilenko AN, Brovarets VS. Chem. Heterocycl. Compd. 2011;47:1020. doi: 10.1007/s10593-011-0869-y. [DOI] [Google Scholar]
- 40.Chumachenko SA, Shablykin OV, Brovarets VS. Chem. Heterocycl. Compd. 1832;2013:49. [Google Scholar]
- 41.O'Neill, L.; Coll, R.; Cooper, M.; Robertson, A.; Schroder, K. WO Patent 2016131098.
- 42.Ye C, Jiao Y, Chiou M-F, Li Y, Bao H. Chem. Sci. 2021;12:9162. doi: 10.1039/D1SC02090K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rios-Solis L, Mothia B, Yi S, Zhou Y, Micheletti M, Lye GJ. J. Mol. Catal. B: Enzym. 2015;120:100. doi: 10.1016/j.molcatb.2015.07.001. [DOI] [Google Scholar]
- 44.Möhrle H, Berlitz J. Pharmazie. 2009;64:565. [PubMed] [Google Scholar]
- 45.Campbell, M. J.; Stevenson, T. M.; Satterfield, A. D. WO Patent 2016182780.
- 46.Declerck D, Josse S, Nguyen Van Nhien A, Szymoniak J, Bertus P, Postel D. Tetrahedron. 2012;68:1145. doi: 10.1016/j.tet.2011.11.067. [DOI] [Google Scholar]
- 47.Tukhtaev HB, Ivanov K, Bezzubov S, Cheshkov D, Melnikov M, Budynina E. Org. Lett. 2019;21:1087. doi: 10.1021/acs.orglett.8b04135. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Zhang H-R, Lin X-R, Yan S-J, Lin J. RSC Adv. 2014;4:27582. doi: 10.1039/C4RA03863K. [DOI] [Google Scholar]
- 49.Orlov VD, Kharchenko YV, Gella IM, Omel'chenko IV, Shishkin OV. Chem. Heterocycl. Compd. 2012;48:1204. doi: 10.1007/s10593-012-1123-y. [DOI] [Google Scholar]
- 50.Fan J, Yang Q-Y, He G-J, Xie X-G, Zhu H-Y, Jin Y, Lin J. RSC Adv. 2014;4:28852. doi: 10.1039/C4RA01966K. [DOI] [Google Scholar]
- 51.Zi Q-X, Yang C-L, Li K, Luo Q, Lin J, Yan S-J. J. Org. Chem. 2020;85:327. doi: 10.1021/acs.joc.9b02063. [DOI] [PubMed] [Google Scholar]
- 52.Fukuyama T, Nakashima N, Okada T, Ryu I. J. Am. Chem. Soc. 2013;135:1006. doi: 10.1021/ja312654q. [DOI] [PubMed] [Google Scholar]
- 53.Jones RCF, Iley JN, Sanchis-Amat M, Zhang X, Elsegood MRJ. Tetrahedron Lett. 2009;50:3577. doi: 10.1016/j.tetlet.2009.03.064. [DOI] [Google Scholar]
- 54.Alizadeh A, Rezvanian A, Deng Y. Tetrahedron. 2010;66:9933. doi: 10.1016/j.tet.2010.10.052. [DOI] [Google Scholar]
- 55.Alizadeh A, Mikaeili A. J. Heterocycl. Chem. 2014;51:527. doi: 10.1002/jhet.1756. [DOI] [Google Scholar]
- 56.Fan, Y.; Liu, S.; Chen, N.; Shao, X.; Xu, X.; Li, Z. Synlett2015, 393.
- 57.Jin J-K, Zhang F-L, Zhao Q, Lu J-A, Wang Y-F. Org. Lett. 2018;20:7558. doi: 10.1021/acs.orglett.8b03303. [DOI] [PubMed] [Google Scholar]
- 58.Murai K, Komatsu H, Nagao R, Fujioka H. Org. Lett. 2012;14:772. doi: 10.1021/ol203313n. [DOI] [PubMed] [Google Scholar]
- 59.Liu, Y.; Wu, J.; Jin, Z.; Jiang, H. Synlett2018, 1061.
- 60.Zhu Z, Lv X, Anesini JE, Seidel D. Org. Lett. 2017;19:6424. doi: 10.1021/acs.orglett.7b03309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J, Liang B, Chen X, Liu Y, Li Y, Liang J, Zhu W, Tang X, Li Y, Zhu Z. Org. Biomol. Chem. 2021;19:4783. doi: 10.1039/D1OB00562F. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Jiang H, Yang J, Jin Z, Chen D. Tetrahedron Lett. 2017;58:546. doi: 10.1016/j.tetlet.2016.12.079. [DOI] [Google Scholar]
- 63.Ibba F, Capurro P, Garbarino S, Anselmo M, Moni L, Basso A. Org. Lett. 2018;20:1098. doi: 10.1021/acs.orglett.8b00009. [DOI] [PubMed] [Google Scholar]
- 64.Ren X, O'Hanlon JA, Morris M, Robertson J, Wong LL. ACS Catal. 2016;6:6833. doi: 10.1021/acscatal.6b02189. [DOI] [Google Scholar]
- 65.Farina C, Gagliardi S, Ghelardini C, Martinelli M, Norcini M, Parini C, Petrillo P, Ronzoni S. Bioorg. Med. Chem. 2008;16:3224. doi: 10.1016/j.bmc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Xie, Y.; Guo, S.; Wu, L.; Xia, C.; Huang, H. Angew. Chem., Int. Ed.2015, 54, 5900. [DOI] [PubMed]
- 67.Zhao X, Liang S, Fan X, Yang T, Yu W. Org. Lett. 2019;21:1559. doi: 10.1021/acs.orglett.8b03927. [DOI] [PubMed] [Google Scholar]
- 68.Liang S, Zhao X, Yang T, Yu W. Org. Lett. 1961;2020:22. doi: 10.1021/acs.orglett.0c00308. [DOI] [PubMed] [Google Scholar]
- 69.Jones, R. C. F.; Rafiq, S.; Elsegood, M. R. J.; McKee, V.; Slater, M. J. Chem.–Asian J.2010, 5, 461. [DOI] [PubMed]
- 70.Jones RCF, Howard KJ, Snaith JS, Blake AJ, Li W-S, Steel PJ. Org. Biomol. Chem. 2011;9:297. doi: 10.1039/C0OB00529K. [DOI] [PubMed] [Google Scholar]
- 71.Jones RCF, Iley JN, Sanchis-Amat M, Zhang X, McKee V, Coles SJ, Gelbrich T. Chem. Commun. 2011;47:7965. doi: 10.1039/c1cc12255j. [DOI] [PubMed] [Google Scholar]
- 72.Evans, K. J.; Campbell, C. L.; Haddow, M. F.; Luz, C.; Morton, P. A.; Mansell, S. M. Eur. J. Inorg. Chem.2019, 4894.
- 73.Evans, K. J.; Mansell, S. M. Chem.–Eur. J.2019, 25, 3766. [DOI] [PMC free article] [PubMed]
- 74.Normand AT, Yen SK, Huynh HV, Hor TSA, Cavell KJ. Organometallics. 2008;27:3153. doi: 10.1021/om800140n. [DOI] [Google Scholar]
- 75.Ye F, Li G-Y, Ding L, Fu Y, Xing Z-Y. Heterocycl. Commun. 2013;19:75. doi: 10.1515/hc-2012-0174. [DOI] [Google Scholar]
- 76.Fu Y, Xu Z-Z, Ye F. Asian J. Chem. 2014;26:2896. doi: 10.14233/ajchem.2014.15959. [DOI] [Google Scholar]
- 77.Ye F, Wang C, Ma P, Zhao L-X, Gao S, Fu Y. J. Heterocycl. Chem. 2018;55:335. doi: 10.1002/jhet.3057. [DOI] [Google Scholar]
- 78.Murata, Y.; Nishikata, T. Chem.–Eur. J.2018, 24, 6354. [DOI] [PubMed]
- 79.Farina, C.; Roletto, J.; Gobbato, S. US Patent 8476453.
- 80.Farina, C.; Roletto, J.; Gobbato, S. WO Patent 2012013640.
- 81.Hoshino Y, Oyaizu M, Koyanagi Y, Honda K. Synth. Commun. 2013;43:2484. doi: 10.1080/00397911.2012.717162. [DOI] [Google Scholar]
- 82.Sharma, S. D. US Patent 7550602.
- 83.Kalyanam N, Rapole KR, Rajendran R, Majeed M. Tetrahedron Lett. 2013;54:5155. doi: 10.1016/j.tetlet.2013.07.009. [DOI] [Google Scholar]
- 84.Kacem Y, Hassine BB. Tetrahedron: Asymmetry. 2014;25:252. doi: 10.1016/j.tetasy.2013.12.002. [DOI] [Google Scholar]
- 85.Cayley, A.; Gallagher, K.; Ménard-Moyon, C.; Schmidt, J.; Diorazio, L.; Taylor, R. Synthesis2008, 3846.
- 86.Dekeukeleire S, D'hooghe M, De Kimpe N. J. Org. Chem. 2009;74:1644. doi: 10.1021/jo802459j. [DOI] [PubMed] [Google Scholar]
- 87.Shavrin KN, Gvozdev VD, Nefedov OM. Mendeleev Commun. 2008;18:300. doi: 10.1016/j.mencom.2008.11.003. [DOI] [Google Scholar]
- 88.Shavrin KN, Gvozdev VD, Nefedov OM. Russ. Chem. Bull. 2010;59:1451. doi: 10.1007/s11172-010-0261-6. [DOI] [Google Scholar]
- 89.Shavrin KN, Gvozdev VD, Nefedov OM. Mendeleev Commun. 2013;23:140. doi: 10.1016/j.mencom.2013.05.006. [DOI] [Google Scholar]
- 90.Gvozdev VD, Shavrin KN, Nefedov OM. Russ. Chem. Bull. 2013;62:2430. doi: 10.1007/s11172-013-0351-3. [DOI] [Google Scholar]
- 91.Liu, S.; Zhang, Z.; Xie, F.; Butt, N. A.; Sun, L.; Zhang, W. Tetrahedron: Asymmetry2012, 23, 329.
- 92.DiRocco DA, Ji Y, Sherer EC, Klapars A, Reibarkh M, Dropinski J, Mathew R, Maligres P, Hyde AM, Limanto J, Brunskill A, Ruck RT, Campeau L-C, Davies IW. Science. 2017;356:426. doi: 10.1126/science.aam7936. [DOI] [PubMed] [Google Scholar]
- 93.Gannedi V, Villuri BK, Reddy SN, Ku C-C, Wong C-H, Hung S-C. J. Org. Chem. 2021;86:4977. doi: 10.1021/acs.joc.0c02888. [DOI] [PubMed] [Google Scholar]
- 94.Wang M, Zhang L, Huo X, Zhang Z, Yuan Q, Li P, Chen J, Zou Y, Wu Z, Zhang W. Angew. Chem., Int. Ed. 2020;59:20814. doi: 10.1002/anie.202011527. [DOI] [PubMed] [Google Scholar]
- 95.Wang M, Zhang X, Ling Z, Zhang Z, Zhang W. Chem. Commun. 2017;53:1381. doi: 10.1039/C6CC09451A. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z, Wang M, Xie F, Sun H, Zhang W. Adv. Synth. Catal. 2014;356:3164. doi: 10.1002/adsc.201400415. [DOI] [Google Scholar]
- 97.Basiak D, Wojciechowski T, Plichta A, Ochal Z, Socha P, Rzepiński P, Dobrzycki Ł, Ziemkowska W. J. Organomet. Chem. 2017;848:302. doi: 10.1016/j.jorganchem.2017.08.009. [DOI] [Google Scholar]
- 98.Ren G, Gong X, Wang B, Chen Y, Huang J. Sep. Purif. Technol. 2015;146:114. doi: 10.1016/j.seppur.2015.03.025. [DOI] [Google Scholar]
- 99.Tseng M-C, Chu Y-H. Chem. Commun. 2010;46:2983. doi: 10.1039/b924286d. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y-L, Tseng M-C, Chu Y-H. Chem. Commun. 2013;49:2560. doi: 10.1039/c3cc39255d. [DOI] [PubMed] [Google Scholar]
- 101.Li H-Y, Chu Y-H. Molecules. 2020;25:104. doi: 10.3390/molecules25010104. [DOI] [Google Scholar]
- 102.Tseng M-C, Chu Y-H. Anal. Chem. 1949;2014:86. doi: 10.1021/ac404011z. [DOI] [PubMed] [Google Scholar]
- 103.Masoodi KZ, Xu Y, Dar JA, Eisermann K, Pascal LE, Parrinello E, Ai J, Johnston PA, Nelson JB, Wipf P, Wang Z. Mol. Cancer Ther. 2017;16:2120. doi: 10.1158/1535-7163.MCT-17-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Subramanian G, Johnson PD, Zachary T, Roush N, Zhu Y, Bowen SJ, Janssen A, Duclos BA, Williams T, Javens C, Shalaly ND, Molina DM, Wittwer AJ, Hirsch JL. ACS Chem. Biol. 2019;14:1205. doi: 10.1021/acschembio.9b00126. [DOI] [PubMed] [Google Scholar]
- 105.Sharma, S. D.; Burris, K. D.; Rajpurohit, R. US Patent 20090076029.
- 106.Burris, K. D.; Sharma, S. D. US Patent 20090081197.
- 107.Sharma, S. D.; Burris, K. D.; Rajpurohit, R. WO Patent 2009144432.
- 108.Sharma, S. D.; Burris, K. D. WO Patent 2009144433.
- 109.Farina, C.; Ghelardini, C.; Petrillo, P. WO Patent 2008058988.
- 110.Farina, C.; Ghelardini, C.; Petrillo, P. US Patent 7973066.