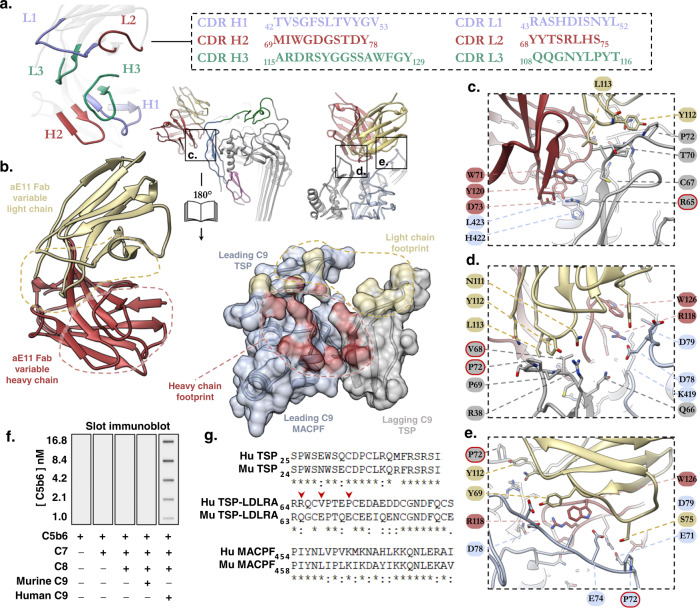
Fig. 2. Structural basis of aE11-Fab/polyC9 binding.
a Illustration of the aE11-CDRs (coloured, blue, green, red) on the antibody and its corresponding primary sequence. b Focused view of the opened aE11-polyC9 interface (split by 180°) that defines the MAC neoepitope. Left: close-up structure of the aE11-CDRs. Right: Transparent surface and cartoon illustration of the leading (light blue) and lagging (grey) C9 subunits, where the footprints of heavy and light chain binding regions are shaded red and yellow, respectively. A dashed line illustrates the buried surfaces of the interface. c–e Key regions of antibody binding to C9. Locations of these regions correspond to the boxed regions in (b). Mutated residues for validation studies are encircled in a red outline. f Slot immunoblot of aE11 binding to the oligomeric C9 component of whole MAC. Oligomeric human C9 is recognised by aE11, but not oligomeric mouse C9. MAC assembly intermediates (C5b6, C5b-7, C5b-8) are included as controls. Concentrations of C5b6 are marked for each condition. The C7, C8αβγ, and C9 concentrations were constant at 30, 30, and 7 nM, respectively. g Sequence alignment of the linear regions of human and murine C9 that contribute to the quaternary discontinuous epitope.