PURPOSE
Organ-sparing therapy for early-stage I/IIA rectal cancer is intended to avoid functional disturbances or a permanent ostomy associated with total mesorectal excision (TME). The objective of this phase II trial was to determine the outcomes and organ-sparing rate of patients with early-stage rectal cancer treated with neoadjuvant chemotherapy followed by transanal excision surgery (TES).
METHODS
This phase II trial included patients with clinical T1-T3abN0 low- or mid-rectal adenocarcinoma eligible for endoscopic resection who were treated with 3 months of chemotherapy (modified folinic acid–fluorouracil-oxaliplatin 6 or capecitabine-oxaliplatin). Those with evidence of response proceeded to transanal endoscopic surgery 2-6 weeks later. The primary end point was protocol-specified organ preservation rate, defined as the proportion of patients with tumor downstaging to ypT0/T1N0/X and who avoided radical surgery.
RESULTS
Of 58 patients enrolled, all commenced chemotherapy and 56 proceeded to surgery. A total of 33/58 patients had tumor downstaging to ypT0/1N0/X on the surgery specimen, resulting in an intention-to-treat protocol-specified organ preservation rate of 57% (90% CI, 45 to 68). Of 23 remaining patients recommended for TME surgery on the basis of protocol requirements, 13 declined and elected to proceed directly to observation resulting in 79% (90% CI, 69 to 88) achieving organ preservation. The remaining 10/23 patients proceeded to recommended TME of whom seven had no histopathologic residual disease. The 1-year and 2-year locoregional relapse-free survival was, respectively, 98% (95% CI, 86 to 100) and 90% (95% CI, 58 to 98), and there were no distant recurrences or deaths. Minimal change in quality of life and rectal function scores was observed.
CONCLUSION
Three months of induction chemotherapy may successfully downstage a significant proportion of patients with early-stage rectal cancer, allowing well-tolerated organ-preserving surgery.
INTRODUCTION
Organ-sparing therapy for early-stage rectal cancer may avoid a permanent ostomy or functional disturbances, commonly known as low anterior resection syndrome (LARS).1,2
CONTEXT
Key Objective
Can 3 months of modified folinic acid–fluorouracil-oxaliplatin 6 (mFOLFOX6)/capecitabine-oxaliplatin (CAPOX) followed by transanal excision surgery be used to treat magnetic resonance imaging-stage cT1-3bN0 rectal cancer?
Knowledge Generated
Induction mFOLFOX6/CAPOX followed by transanal excision surgery was well tolerated and resulted in downstaging to ypT0/T1 cN0 tumors in 57% of 58 enrolled patients with well to moderately differentiated adenocarcinoma and preserved mismatch repair. Overall, 79% of patients pursued an organ-sparing strategy, with two patients experiencing a locoregional relapse during the 15.4-month follow-up period. Quality of life and rectal function scores demonstrated almost no change compared with baseline.
Relevance
Early results suggest that this novel treatment strategy leads to downstaging to ypT0/T1 cN0 in the majority of selected patients with early rectal cancer. The approach offers a much-desired organ-sparing option and warrants further investigation.
Current standard therapy for patients with histologically high-risk clinical (c) T1 and cT2N0 rectal tumors is oncologic resection with total mesorectal excision (TME), combined with preoperative chemoradiation for patients with T3 or N1 tumors.3 Although locoregional relapse rates with modern neoadjuvant therapy are low and survival is excellent, TME results in issues with bowel function, incontinence, and sexual function. The addition of perioperative radiation can result in LARS in up to 60% of patients.1,4,5
Transanal excision surgery (TES) is increasingly used for treatment of select T1N0 or T2N0 rectal tumors.6,7 Nonetheless, a significant proportion of cT1-2N0 tumors are pathologically node-positive and there is an increased rate of local relapse with local excision compared with surgical resection.8-10 Multiple, single-arm and randomized phase II studies exploring the use of pelvic chemoradiation followed by TES in patients with cT1-3 rectal cancer have reported an organ preservation rate of 50%-68%.11-15 Unfortunately, preoperative radiation significantly increases wound-healing complications16 and adversely affects sphincter and sexual function.17
Neoadjuvant chemotherapy with excision is a potential approach to reduce local and distal relapse of rectal tumors, reducing locoregional recurrence in stage II/III rectal cancer.18 There is no prospective experience of neoadjuvant chemotherapy and transanal surgery for stage I rectal tumors. The Canadian Cancer Trials Group (CCTG) CO.28 NEO phase II trial was designed to determine the outcomes and organ preservation rate of the patients with cT1-T3abN0 rectal tumors treated with chemotherapy (modified folinic acid–fluorouracil-oxaliplatin 6 [mFOLFOX6] or capecitabine-oxaliplatin [CAPOX]) followed by transanal surgery and explore the prognostic value of tumor biomarkers for the outcomes.
METHODS
Study Design and Participants
This phase II, nonrandomized, open-label trial accrued adult patients with invasive, well-moderately differentiated rectal adenocarcinoma, stage cT1-T3ab and node-negative (cN0) at seven institutions in Canada and the United States. Patients had low- or mid-rectal tumors by proctoscopy and were deemed eligible for endoscopic resection by the study surgeon. All patients required pelvic magnetic resonance imaging (MRI), computed tomography (CT) scan of the chest, abdomen, and pelvis.
Further criteria included a pretreatment Eastern Collaborative Oncology Group performance score of 0 or 1 and adequate hematologic and organ function. Exclusion criteria included a history of external-beam pelvic radiation, prior therapy for rectal cancer, or metastatic disease.
The study was approved by the site institutional review board, and all patients provided written informed consent to participate.
Procedures
Patients commenced therapy with six cycles of mFOLFOX6 or four cycles of CAPOX, on the basis of investigator discretion. Each 14-day cycle of mFOLFOX6 consisted of leucovorin 400 mg/m2 and oxaliplatin 85 mg/m2 in one 2-hour infusion, bolus fluorouracil 400 mg/m2 once on day 1, and one 46-hour infusion of fluorouracil 2,400 mg/m2. Each 21-day cycle of CAPOX consisted of capecitabine 1,000 mg/m2 twice daily for 14 days, and oxaliplatin 130 mg/m2 once on day 1.
Postchemotherapy pelvic MRI and proctoscopy were performed 2-3 weeks after the last dose of chemotherapy. Tumors with protocol-defined evidence of response proceeded to TES. Those with progression or no response to chemotherapy were recommended TME surgery and preoperative pelvic radiation if the MRI revealed cT3ab, cN+, or involved or threatened circumferential radial margin.
TES was performed between 2-6 weeks after the last cycle of chemotherapy. Tumor excision included a minimum of 1-cm gross margin.19 Trial surgeons had performed at least 20 previous TES procedures for rectal cancer before commencement of the study,20-22 and each surgeon was required to submit an unedited video of a TES procedure with sutured defect closure for central review. TES could be performed with transanal minimally invasive surgery or transanal endoscopic microsurgery platform or via open transanal excision, per surgeon preference and tumor location.
Local pathology yp (pathologic stage following systemic or radiation therapy prior to surgery) staging review determined subsequent therapy. Patients with yp stage T0/N0 or T1N0 with no poor prognostic features (T1 good) were recommended observation, whereas ypT1 tumors with poor prognostic features, ypT2/3, or any N+ were recommended radical TME surgery. Poor prognostic features included poorly differentiated histology, lymphovascular invasion, and/or positive margin within < 1 mm. Patients assigned to observation were followed for 36 months from the time of TES with proctoscopy every 6 months, pelvic MRI every 6 months (could be substituted with pelvic CT at months 12, 24, and 36), carcinoembryonic antigen every 6 months, and annual contrast CT of the chest, abdomen, and pelvis for 3 years.
Adverse events during chemotherapy were measured using the Common Terminology Criteria for Adverse Events Version 5.0. Perioperative and operative details were collected prospectively. Quality of life (QoL) and rectal function was assessed using European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–C30, Fecal Incontinence Quality of Life (FIQL),23 and the LARS surveys.24 Questionnaires were administered at baseline, pre-excision (postchemotherapy), and 6, 12, 24, and 36 months after tumor surgery.
Molecular profiling was performed on pretreatment formalin-fixed, paraffin-embedded diagnostic biopsies where sufficient tumor was available. If there was inadequate tumor DNA from the diagnostic biopsy, resection specimens were used. Next-generation sequencing (NGS) using the FoundationOne CDx assay was conducted at Foundation Medicine (Cambridge, MA) following standard procedures. This US Food and Drug Administration–approved companion diagnostic provides information on all coding exons from 309 cancer-related genes, fusion identification of 34 commonly rearranged genes, microsatellite instability, and tumor mutation burden (TMB).25,26
Statistical Analysis
The primary end point was protocol-specified organ preservation rate (psOPR), defined as the proportion of patients with tumor downstaging to ypT0/T1goodN0 and who avoided radical surgery. On the basis of the literature published at the time, an organ-preservation rate of 50%-68% would be expected among patients with cT1-3N0 rectal tumors treated with chemoradiation and transanal endoscopic surgery.11-15 On the basis of this experience, the experimental treatment would be considered of no interest if the psOPR were 50% or lower (H0) and as promising if it were 65% or higher (H1).
The study used a two-stage minimax design with a plan to stop the trial at the end of first stage if ≤ 10 of the first 22 patients accrued met the protocol specified criteria of organ preservation. The experimental procedure would be considered as promising if, out of the total patients accrued, 30 or more met the protocol specified criteria of organ preservation. The type-I error rate and power of this design was 0.1 and 0.8, respectively. Minimum and maximum sample sizes, respectively, were 22 and 50 evaluable patients. Allowing for up to 15% of patients who may not commence neoadjuvant chemotherapy and be treated with surgery and thus be considered as inevaluable, the total sample size was 58 patients.
The psOPR was estimated using the intention-to-treatment principle for all patients completing neoadjuvant chemotherapy. Because of the observation that a high proportion of patients declined protocol recommended surgery, the observed organ preservation rate (oOPR) was defined at the time of data analysis, representing the proportion of patients downstaged to ypT0/T1 plus higher yp stage patients who declined recommended TME surgery. Exact Clopper-Pearson confidence intervals were calculated for both psOPR and oOPR. Kaplan-Meier method was used to estimate 1- and 2-year locoregional relapse-free survival, from the date of enrollment. Patients were censored at latest date of last date of distant relapses (for locoregional relapse-free survival only), date of death, date of lost to follow-up, and last disease assessment date.
Adverse events were analyzed for treated patients. The means of QoL and rectal function scales at each time point were calculated. European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–C30 functioning and global scales were scored from 0 to 100, with higher scores indicating better QoL. FIQL was scored with a range from 1 to 5, with 1 indicating a lower functional status of QoL.23 Proportion of patients with LARS scores in the following validated categories was calculated at each time point: 0-20 no LARS, 21-29 minor LARS, and 30-42 major LARS.24 The prognostic value of RAS, the main variable of interest in genomic analyses, was evaluated by a Fisher's exact test with a comparison of the psOPR between patients with mutated and wild-type RAS.
The study was conducted by the Canadian Cancer Trials Group with support from the Canadian Cancer Society. The trial is registered with ClinicalTrials.gov (identifier: NCT03259035).27
RESULTS
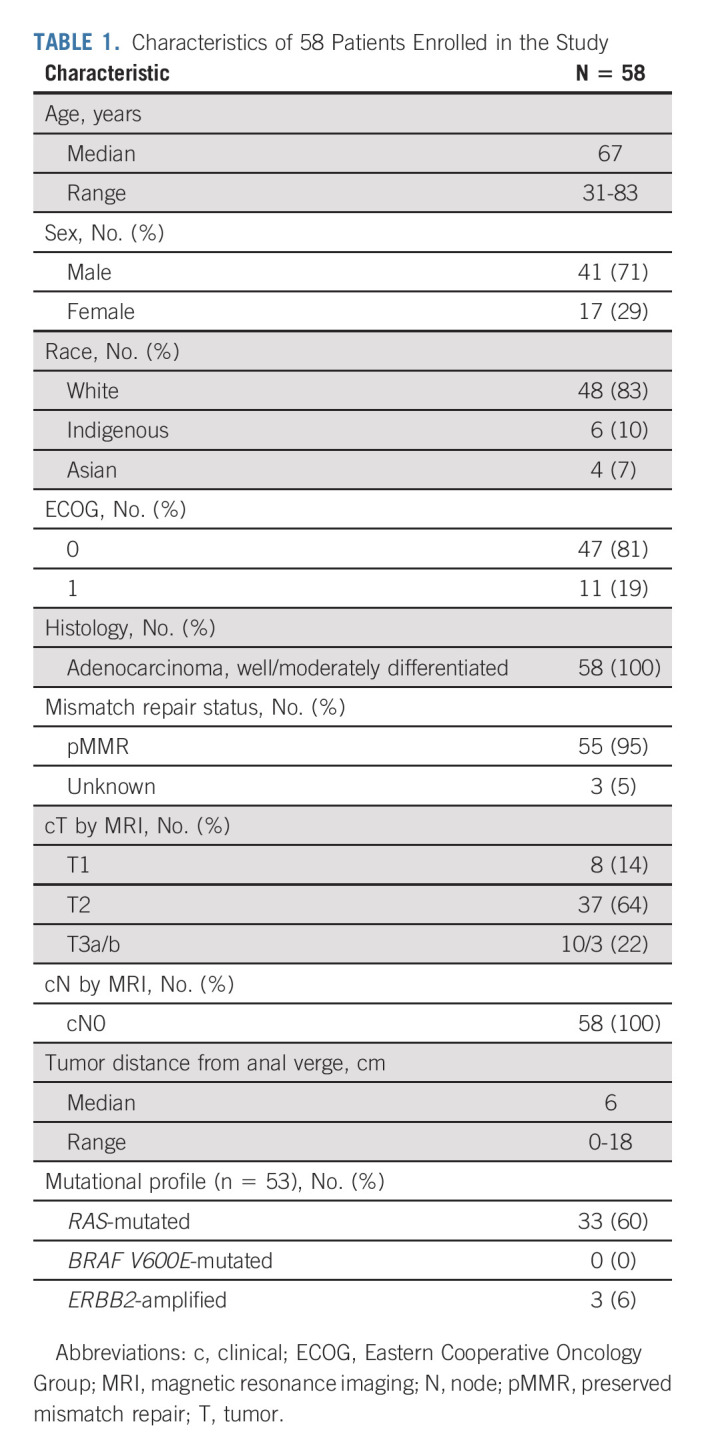
The study opened to accrual on August 22, 2017. After enrollment of the initial 22 patients, the interim efficacy analysis on April 19, 2019, documented that > 50% of patients avoided radical surgery, allowing full accrual to be completed on May 19, 2020. The results presented are based on database lock from January 20, 2021, and a median follow-up of 15.4 months. A total of 58 eligible patients were accrued in Canada and the United States and characteristics are summarized in Table 1. Patients had a median age of 67 years, 71% were male, and all had well-moderately differentiated, nonmucinous rectal adenocarcinoma. Most common MRI cT-stage was T2 (64%), and the median tumor height was 6.0 (range, 0-18) cm.
TABLE 1.
Characteristics of 58 Patients Enrolled in the Study
Chemotherapy
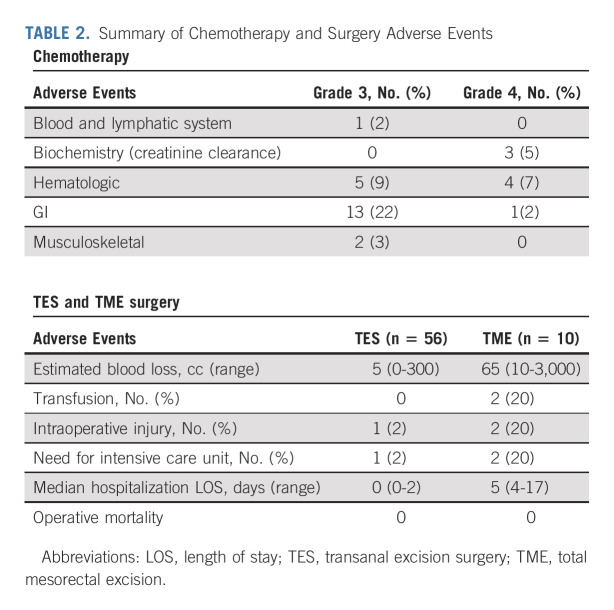
All 58 enrolled patients commenced either mFOLFOX6 (32) or CAPOX (26) of which 91% and 89%, respectively, completed all planned cycles. Oxaliplatin relative dose intensity (RDI) was ≥ 90% in 59%, 80% to < 90% in 22%, and 60%-80% in 19%. Infusional 5-fluorouracil RDI was ≥ 90% in 53%, 80% to < 90% in 22%, and 60%-80% in 19%, whereas RDI for capecitabine was reduced at 80% to < 90% in 50%, 60% to < 80% in 22%, and < 60% in 19%. There were no unexpected toxicities; the most common grade 3/4 adverse events are summarized in Table 2, and there were no grade 5 events.
TABLE 2.
Summary of Chemotherapy and Surgery Adverse Events
Surgical Therapy
Among 58 patients who commenced chemotherapy, 56 proceeded to TES by transanal minimally invasive surgery/transanal endoscopic microsurgery (53), or open local excision (three), of whom 52 (93%) had an R0 excision, two (3.5%) were R1, and two (3.5%) R2. Of the 56 patients who had a tumor excision, 10 proceeded to subsequent protocol-recommended TME surgery on the basis of residual ypT2+ and/or R1/2 tumor in the excision specimen. Morbidity of TES and TME surgeries was low (Table 2).
Two of the 58 enrolled patients did not proceed to TES after completion of chemotherapy. One, who had an initial cT1N0 tumor and developed progression to cT3b on imaging after chemotherapy, proceeded directly to TME surgery. TME pathologic specimen showed ypT2N0 with a negative circumferential margin. The second patient with initial cT2N0 tumor had no chemotherapy response, and was treated with pelvic chemoradiation at standard doses with a clinical tumor response and scheduled for organ-sparing TES because of patient preference.
Organ Preservation
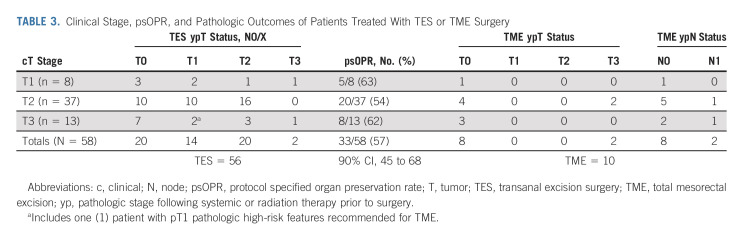
The psOPR is presented in an intention-to-treat analysis in Table 3. A total of 33/58 patients had tumor downstaging to ypT0/1N0/X on the TES specimen, resulting in a psOPR of 57% (90% CI, 45 to 68). The psOPR was similar among T stage subgroups and was 63% (n = 8), 54% (n = 37), and 62% (n = 13) in cT1, T2, and T3ab groups, respectively. Of 23 remaining patients recommended for TME surgery on the basis of protocol requirements, 13 declined and elected to proceed directly to observation resulting in an oOPR of 46/58 (79% with 90% CI, 69 to 88). The yp tumor stage on TES specimen of the 13 patients who declined TME was T1 with histologic high-risk features in one patient, T2 in 11 patients, and T3 in one patient. The final TME specimen yp stage of the 10 patients who proceeded to surgery is provided (Table 3), of whom seven had no pathologically residual disease.
TABLE 3.
Clinical Stage, psOPR, and Pathologic Outcomes of Patients Treated With TES or TME Surgery
Summary of Therapy
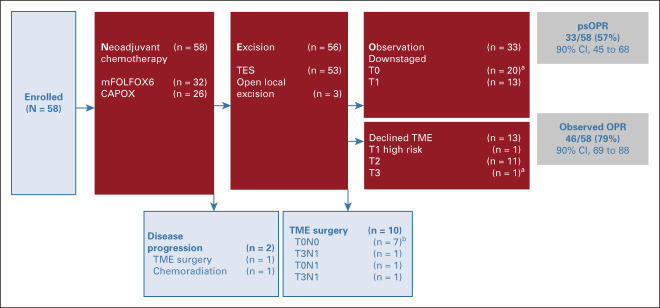
Treatment of all enrolled patients is summarized in a flow diagram in Figure 1. During the follow-up period, two locoregional recurrences were documented, one in a patient who declined protocol recommended TME surgery. Both were initially diagnosed with MRI-stage cT2, low-lying tumors at enrollment. One had residual ypT2, whereas the other had ypT1 on TES specimen after induction chemotherapy. The 1-year and 2-year locoregional relapse-free survival rates were, respectively, 98% (95% CI, 86 to 100) and 90% (95% CI, 58 to 98; see Appendix Fig A1 [online only]). There were no distant recurrences or deaths.
FIG 1.
Flow diagram of 58 patients enrolled in the study. aRepresents ypT, cN0 stage. bRepresents yp stage. c, clinical; CAPOX, capecitabine-oxaliplatin; mFOLFOX, modified folinic acid–flurouracil-oxaliplatin 6; N, node; oOPR, observed organ preservation rate; psOPR, protocol-specified organ preservation rate; T, tumor; TES, transanal excision surgery; TME, total mesorectal excision; yp, pathologic stage following systemic or radiation therapy prior to surgery.
Tumor Genotyping
Tumor genotyping with FoundationOne CDx was done for 53/58 patients with sufficient tissue. Median TMB (interquartile range) was 2.52 mutations/Mb (1.26-5.04) in the 48 patients with TMB available, and the frequency of the most common variants is provided in Figure 2 for 53 patients with sufficient tissue for NGS. There was no association between RAS mutation status and tumor downstaging after mFOLFOX6/CAPOX chemotherapy with a total of 16/33 (49%) of RAS-mutant tumors downstaged to ypT0/1 compared with 12/20 (60%) in the RAS wild-type group, P = .57. No BRAF V600E mutations were noted. MMR status was available in 55/58 patients on the basis of immunohistochemistry (IHC) or NGS assay. 55/55 (100%) were pMMR, and concordance between IHC and NGS was 30/30 (100%) in patients with overlapping assays. ERBB2 amplification was noted in 3/53 patients (5.7%), with all three showing tumor downstaging and a psOPR of 67%.
FIG 2.
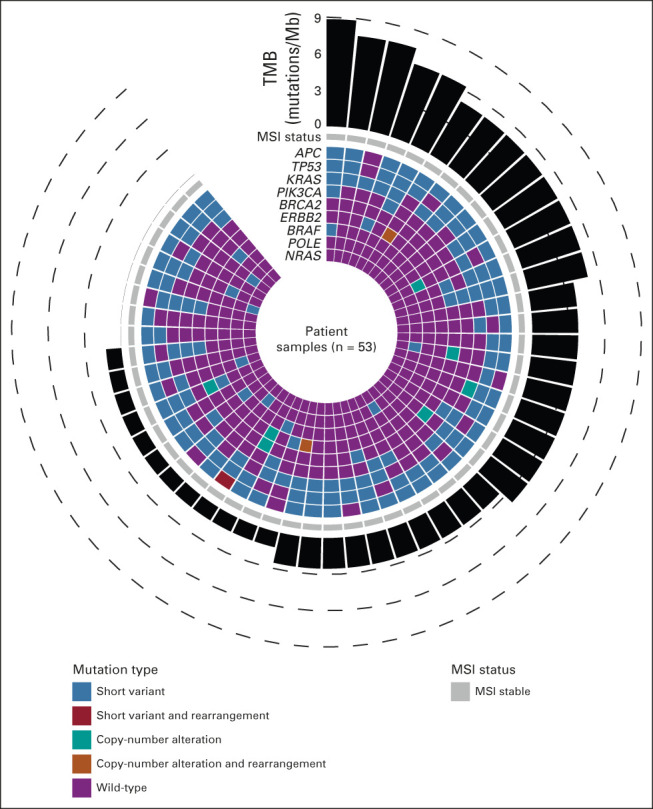
Tumor genotype by FoundationOne CDx analysis in 53 patients with sufficient archival tumor tissue. MSI, microsatellite; TMB, tumor mutation burden.
Compliance with all QoL and rectal function instruments was 100% at baseline, 89% pre-excision, 85% at 6 months, and 86% at 12 months (Figs 3A-3C). At baseline, the proportion of patients with major LARS was 10%. No patient had major LARS at pre-excision visit. The proportion of patients with major LARS was 22% at 6 months postexcision and was decreased to 14% at 12 months. Total mean LARS scores are provided in Figure 3B. At all time points, there was almost no change from baseline for FIQL lifestyle and depression/self-perception scales (Fig 3C).
FIG 3.
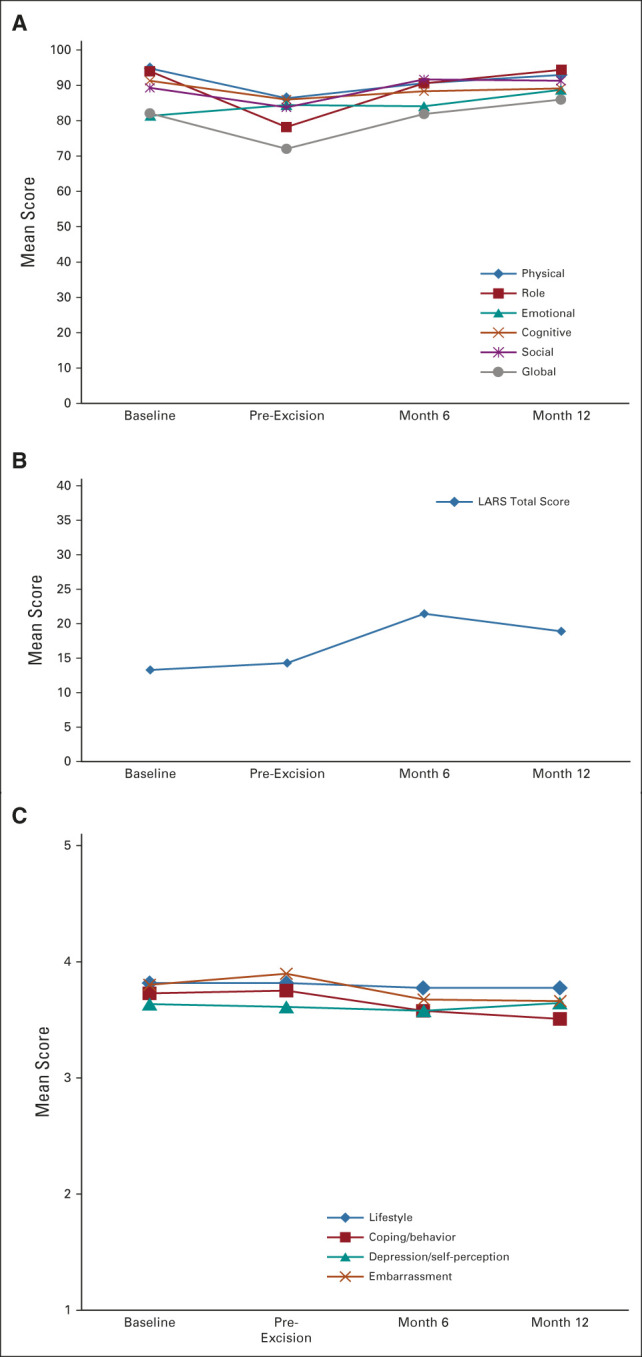
(A) EORTC QLQ-C-30 scores at baseline, pre-excision, 6 months, and 12 months after TES. Functioning and global scales were scored from 0 to 100, with higher scores indicating better QoL. Observed/expected sample size was 58/58 at baseline, 53/58 at pre-excision, 37/43 at 6 months after TES, and 23/26 at 12 months after TES. (B) LARS mean total scores at baseline, pre-excision, 6 months, and 12 months after excision. Scores of 0-20 correspond to no LARS, 21-29 to minor LARS, and 30-42 to major LARS. Observed/expected sample size was 58/58 baseline, 50/58 pre-excision, 36/43 at 6 months after TES, and 21/26 at 12 months after TES. (C) FIQL mean scores at baseline, pre-excision, 6 months, and 12 months after excision. FIQL was scored with a range from 1 to 5 with 1 indicating a lower functional status of QoL. Observed/expected sample size was 52/58 at baseline, 52/58 at pre-excision, 37/43 at 6 months after TES, and 23/26 at 12 months after TES. EORTC QLQ-C-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–C30; FIQL, Fecal Incontinence Quality of Life; LARS, Low Anterior Resection Syndrome; QoL, quality of life; TES, transanal excision surgery.
The video presentation of the study results presented at Virtual ASCO 2021 are provided in the Data Supplement (online only).
DISCUSSION
To our knowledge, this is the first published study to describe organ-sparing outcomes of patients with stage I and early-stage II rectal cancer treated with induction chemotherapy and transanal surgical excision. More than half of the enrolled patients experienced complete or near-complete pathologic downstaging and met the protocol criteria for organ-sparing therapy. A significantly larger proportion of patients chose to defer recommended TME surgery in favor of close observation on study. No unexpected toxicities were encountered.
Study results compare favorably to prior organ-sparing studies using standard chemoradiation before TES, including the CARTS trial, in which 55% of 55 patients with low T1-T3abN0 rectal tumors achieved tumor downstaging to ypT0/1.13,14 In the ACOSOG Z6041, downstaging to ypT0/T1 was achieved in 64% of 77 patients with cT2N0 tumors treated with chemoradiation with capecitabine and oxaliplatin,12 whereas in the GRECCAR2 trial, 64% (n = 74) of patients with cT2- 3/N0-1 tumors downstaged to ypT0/1,15 and Lezoche et al11 reported 52% of cT2N0 tumors (n = 100) were ypT0/1 stage after chemoradiation.
The oOPR was 79%, including patients who declined recommended surgery, and may reflect the patient preference to avoid radical surgery despite a higher risk of locoregional recurrence. This choice in favor of an organ-sparing approach is similar to trends observed in other prospective studies.11,13,14 Among the 13 patients who declined recommended TME, 85% had ypT2N0 tumors and one patient developed a locoregional relapse during the follow-up period that was successfully resected with a TME. Among the 10 patients who proceeded to recommended TME surgery, only two had pathologically N1 tumors (Table 3), a rate similar to the expected node-positive rate of pT2 tumors described in prior literature.8-10
Only one of 58 patients enrolled was treated with pelvic chemoradiation in this study. This patient had no response to chemotherapy documented by pre-excision MRI and endoscopy; however, a significant tumor response was achieved after subsequent standard long-course chemoradiation, after which the patient was eligible for organ-sparing TES.
In this protocol, surgery followed chemotherapy, in contrast to the watch-and-wait approach according to previous published criteria used for stage II/III rectal tumors.28 This study demonstrates the important role played by TES in accurately documenting the residual tumor burden, thereby informing provider and patient decision making about the need of subsequent therapy. In the 13/56 (23%) of patients who underwent TES with a resultant ypT1 tumor, transanal surgery removed microscopic residual disease that may otherwise have been difficult to differentiate from a clinical complete response on proctoscopy and MRI. Furthermore, recent work suggests TES can enhance the opportunity for organ preservation even in patients with locally advanced disease who achieve a near-complete response.29
In this study, there were very few postoperative complications and no patients required readmission after TES. The early QoL results suggest that TES had only minor effects on bowel function, which subsequently improved. This is in line with prior published reports that anal manometric values return to normal by 6-12 months, and continence scores back to normal by 1 year in TES patients.30 In this study, mean LARS score increased to minor LARS 6 months after excision and improved by the 12-month time point to no LARS, similar to baseline. Minimal change in fecal incontinence scores was noted on the FIQL questionnaire.
Molecular testing for biomarkers was successfully done on most patients. The common alteration, RAS mutation, was not associated with response to chemotherapy. Tumors had preserved mismatch repair, a low mutation burden, and no BRAF V600E mutations, characteristic of the majority of left-sided colorectal tumors.31 ERBB2 gene amplification was documented in 6% of tumors (three) having a yp stage of T0, T1, and T2 after chemotherapy. Given the small number of patients with this alteration, it is difficult to make any firm statements about predictive efficacy in this population.
The study included patients with MRI-staged early rectal cancer with no unfavorable histologic characteristics. A larger, hypothesis-confirming study is required in this patient population. Patients were treated at cancer centers specialized in rectal cancer diagnosis, therapy, and transanal surgery. This concentration of rectal cancer surgery at higher-volume centers has been previously described32 with consequent superior outcomes in the setting of organ-sparing therapy. The American College of Surgeons (2014) created the National Accreditation Program for Rectal Cancer structured around evidence-based and a multidisciplinary team approaches,33 a strategy enabling patients to benefit from novel approaches such as NEO.
In conclusion, three months of induction chemotherapy with mFOLFOX6/CAPOX may successfully downstage a significant proportion of patients with favorable-risk, early-stage rectal cancer, allowing well-tolerated organ-preserving surgical therapy with minimal effect on organ function.
APPENDIX
FIG A1.
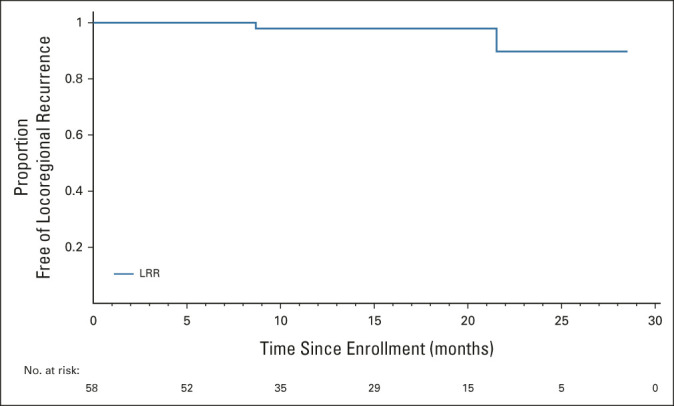
Kaplan-Meier curve for LRR-free survival among 58 patients enrolled in the study. LRR, locoregional recurrence.
Hagen F. Kennecke
Honoraria: Natera, Tersera, Novartis
Consulting or Advisory Role: Tersera
Speakers' Bureau: Natera
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Exelixis (Inst)
Jonathan M. Loree
Consulting or Advisory Role: Taiho Pharmaceutical, Ipsen, Novartis, Bayer, Amgen, Eisai, Pfizer
Research Funding: Ipsen (Inst), Amgen (Inst)
Hussein Moloo
Honoraria: Johnson and Johnson
Rebecca Auer
Research Funding: Qu Biologics (Inst)
Patents, Royalties, Other Intellectual Property: US201662372406P: Oncolytic rhabdovirus expressing IL12, U.S. Provisional Patent Application No. 63/165,539 filed March 24, 2021, and titled Perioperative Innate Immune Priming in Cancer Therapy
Uncompensated Relationships: Immodulon Therapeutics
Manoj Raval
Consulting or Advisory Role: Northgate Technologies Inc
Research Funding: Cook Myosite (Inst), Cook BioTech (Inst)
Antonio Caycedo-Marulanda
Speakers' Bureau: MINOG
Vlad V. Simianu
Consulting or Advisory Role: C-SATs, Inc
Travel, Accommodations, Expenses: Intuitive Surgical
Open Payments Link: https://openpaymentsdata.cms.gov/physician/4220255
Lacey D. Pitre
Honoraria: Merck, AstraZeneca (Inst), AstraZeneca
Vallerie L. Gordon
Consulting or Advisory Role: AAA Canada consulting, Knight Therapuetics, Incyte Canada
Halla Nimeiri
Employment: Foundation Medicine, Northwestern Medicine
Stock and Other Ownership Interests: Foundation Medicine, AbbVie
Carl J. Brown
Honoraria: Ethicon/Johnson & Johnson
Other Relationship: Medtronic (Inst)
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 173
SUPPORT
Supported by the Canadian Cancer Society (grant no. 707213).
PRIOR PRESENTATION
Presented in part at the 2021 ASCO Annual Meeting, virtual, June 4-8, 2021.
CLINICAL TRIAL INFORMATION
NCT03259035 (NEO)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.00184.
AUTHOR CONTRIBUTIONS
Conception and design: Hagen F. Kennecke, Chris J. O'Callaghan, Hussein Moloo, Rebecca Auer, Derek J. Jonker, Vallerie L. Gordon, Dongsheng Tu, Carl J. Brown
Administrative support: Chris J. O'Callaghan, Katerina Neumann, Max Sherry
Provision of study materials or patients: Derek J. Jonker, Sunil Patel, Ramzi Helewa, Vallerie L. Gordon, Katerina Neumann
Collection and assembly of data: Hagen F. Kennecke, Chris J. O'Callaghan, Jonathan M. Loree, Derek J. Jonker, Manoj Raval, Reilly Musselman, Grace Ma, Antonio Caycedo-Marulanda, Vlad V. Simianu, Sunil Patel, Lacey D. Pitre, Vallerie L. Gordon, Katerina Neumann, Max Sherry, Dongsheng Tu, Carl J. Brown
Data analysis and interpretation: Hagen F. Kennecke, Chris J. O'Callaghan, Jonathan M. Loree, Derek J. Jonker, Manoj Raval, Vlad V. Simianu, Ramzi Helewa, Vallerie L. Gordon, Halla Nimeiri, Dongsheng Tu, Carl J. Brown
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Chemotherapy, Excision, and Observation for Early Rectal Cancer: The Phase II NEO Trial (CCTG CO.28) Primary End Point Results
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Hagen F. Kennecke
Honoraria: Natera, Tersera, Novartis
Consulting or Advisory Role: Tersera
Speakers' Bureau: Natera
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Exelixis (Inst)
Jonathan M. Loree
Consulting or Advisory Role: Taiho Pharmaceutical, Ipsen, Novartis, Bayer, Amgen, Eisai, Pfizer
Research Funding: Ipsen (Inst), Amgen (Inst)
Hussein Moloo
Honoraria: Johnson and Johnson
Rebecca Auer
Research Funding: Qu Biologics (Inst)
Patents, Royalties, Other Intellectual Property: US201662372406P: Oncolytic rhabdovirus expressing IL12, U.S. Provisional Patent Application No. 63/165,539 filed March 24, 2021, and titled Perioperative Innate Immune Priming in Cancer Therapy
Uncompensated Relationships: Immodulon Therapeutics
Manoj Raval
Consulting or Advisory Role: Northgate Technologies Inc
Research Funding: Cook Myosite (Inst), Cook BioTech (Inst)
Antonio Caycedo-Marulanda
Speakers' Bureau: MINOG
Vlad V. Simianu
Consulting or Advisory Role: C-SATs, Inc
Travel, Accommodations, Expenses: Intuitive Surgical
Open Payments Link: https://openpaymentsdata.cms.gov/physician/4220255
Lacey D. Pitre
Honoraria: Merck, AstraZeneca (Inst), AstraZeneca
Vallerie L. Gordon
Consulting or Advisory Role: AAA Canada consulting, Knight Therapuetics, Incyte Canada
Halla Nimeiri
Employment: Foundation Medicine, Northwestern Medicine
Stock and Other Ownership Interests: Foundation Medicine, AbbVie
Carl J. Brown
Honoraria: Ethicon/Johnson & Johnson
Other Relationship: Medtronic (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Chen TY, Wiltink LM, Nout RA, et al. : Bowel function 14 years after preoperative short- course radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomized trial. Clin Colorectal Cancer 14:106-114, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Sturiale A, Martellucci J, Zurli L, et al. : Long-term functional follow-up after anterior rectal resection for cancer. Int J Colorectal Dis 32:83-88, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, Venook AP, Al-Hawary MM, et al. : NCCN guidelines insights: Rectal cancer, version 6.2020. J Natl Compr Canc Netw 18:806-815, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Battersby NJ, Juul T, Christensen P, et al. : Predicting the risk of bowel-related quality-of-life impairment after restorative resection for rectal cancer: A multicenter cross-sectional study. Dis Colon Rectum 59:270-280, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Ihnát P, Slívová I, Tulinsky L, et al. : Anorectal dysfunction after laparoscopic low anterior rectal resection for rectal cancer with and without radiotherapy (manometry study). J Surg Oncol 117:710-716, 2018 [DOI] [PubMed] [Google Scholar]
- 6.You YN, Baxter NN, Stewart A, Nelson H: Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: A nationwide cohort study from the National Cancer Database. Ann Surg 245:726-733, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stitzenberg KB, Sanoff HK, Penn DC, et al. : Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol 31:4276-4282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minsky BD, Rich T, Recht A, et al. : Selection criteria for local excision with or without adjuvant radiation therapy for rectal cancer. Cancer 63:1421-1429, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Brodsky JT, Richard GK, Cohen AM, Minsky BD: Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer 69:322-326, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Salinas HM, Dursun A, Klos CL, et al. : Determining the need for radical surgery in patients with T1 rectal cancer. Arch Surg 146:540-543, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Lezoche E, Baldarelli M, Lezoche G, et al. : Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 99:1211-1218, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Aguilar J, Shi Q, Thomas CR, Jr, et al. : A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: Preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 19:384-391, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verseveld M, de Graaf EJ, Verhoef C, et al. : Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg 102:853-860, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Stijns RCH, de Graaf EJR, Punt CJA, et al. : Long-term oncological and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer: The CARTS study. JAMA Surg 154:47-54, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rullier E, Vendrely V, Asselineau J, et al. : Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol 5:465-474, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Marks JH, Valsdottir EB, DeNittis A, et al. : Transanal endoscopic microsurgery for the treatment of rectal cancer: Comparison of wound complication rates with and without neoadjuvant radiation therapy. Surg Endosc 23:1081-1087, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hupkens B, Lambregts D, Van der Sande M, et al. : 2001 Anorectal function after watch and wait-policy in rectal cancer patients. Eur J Cancer 51:S327, 2015 [Google Scholar]
- 18.Bosset JF, Collette L, Calais G, et al. : Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114-1123, 2006. [Erratum: N Engl J Med 357:728, 2007] [DOI] [PubMed] [Google Scholar]
- 19.Monson JR, Weiser MR, Buie WD, et al. : Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 56:535-550, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Stevenson AR, Solomon MJ, Lumley JW, et al. : Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomized clinical trial. JAMA 314:1356-1363, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Bonjer HJ, Deijen CL, Abis GA, et al. : A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324-1332, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Fleshman J, Branda M, Sargent DJ, et al. : Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: The ACOSOG Z6051 randomized clinical trial. JAMA 314:1346-1355, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood TH, Church JM, Fleshman JW, et al. : Fecal incontinence quality of life scale: Quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 43:9-17, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Juul T, Ahlberg M, Biondo S, et al. : International validation of the low anterior resection syndrome score. Ann Surg 259:728-734, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marabelle A, Fakih M, Lopez J, et al. : Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353-1365, 2020 [DOI] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov : NEO: Neoadjuvant Chemotherapy, Excision and Observation for Early Rectal Cancer. Bethesda, MD, National Library of Medicine (US), 2000. https://clinicaltrials.gov/ct2/show/NCT03259035?id=NCT03259035&draw=2&rank=1&l oad=cart [Google Scholar]
- 28.Smith JJ, Strombom P, Chow OS, et al. : Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol 5:e185896, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones HJS, Al-Najami I, Baatrup G, Cunningham C: Local excision after (near) complete response of rectal cancer to neoadjuvant radiation: Does it add value? Int J Colorectal Dis 36:1017-1022, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Allaix ME, Arezzo A, Morino M: Transanal endoscopic microsurgery for rectal cancer: T1 and beyond? An evidence-based review. Surg Endosc 30:4841-4852, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Network : Comprehensive molecular characterization of human colon and rectal cancer. Nature 487:330-337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Aquina CT, Justiniano CF, et al. : Centralizing rectal cancer surgery: What is the impact of travel on patients? Dis Colon Rectum 63:319-325, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Brady JT, Xu Z, Scarberry KB, et al. : Evaluating the current status of rectal cancer care in the US: Where we stand at the start of the commission on cancer's National Accreditation Program for Rectal Cancer. J Am Coll Surg 226:881-890, 2018. [Erratum: J Am Coll Surg 227:484-487, 2018] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.00184.