Figure 2. TIL and TCR-T cell therapy cell product generation, patient treatment, and mechanisms of tumor engagement.
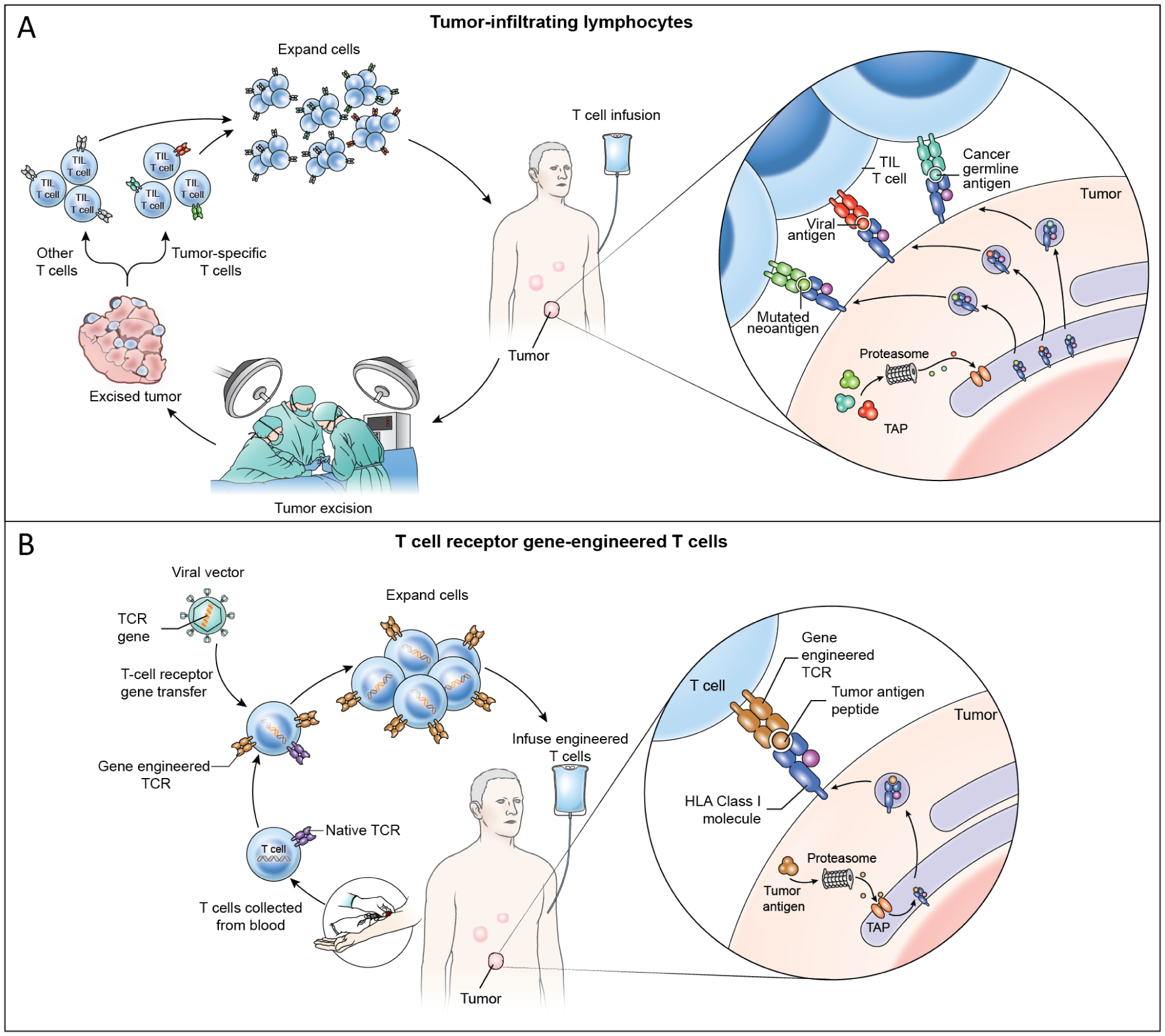
(A) In TIL therapy, a metastatic tumor is surgically resected and T cells are grown from the tumor ex vivo. The T cells are oligoclonal and may or may not target tumor antigens and may be composed of a mixture of tumor-specific T cells and other T cells. The T cells are expanded ex vivo and administered by intravenous infusion. TIL treatments resulting in durable, complete regression of cervical cancer were mediated by targeting of HPV oncoproteins as well as non-viral proteins such as mutated neoantigens and cancer germline antigens (cutout).19 Case reports have shown partial responses to TIL therapy solely targeting mutated neoantigens in non-viral epithelial cancers.20,28 (B) In TCR-T cell therapy, T cells are collected from peripheral blood and gene-engineered ex vivo to express a defined tumor-antigen-targeting TCR. TCR gene engineering may be conducted with a viral vector (shown) or with other gene transfer methods such as CRISPR-CAS9 gene editing or transposon systems. Engineered TCR-T cells are expanded ex vivo and administered intravenously. TCR-T cells engage tumors through recognition of peptide HLA complex composed of a defined peptide from a tumor antigen (cutout).
