Table 2:
Scope of Iron-catalyzed decarboxylative protonation[a]
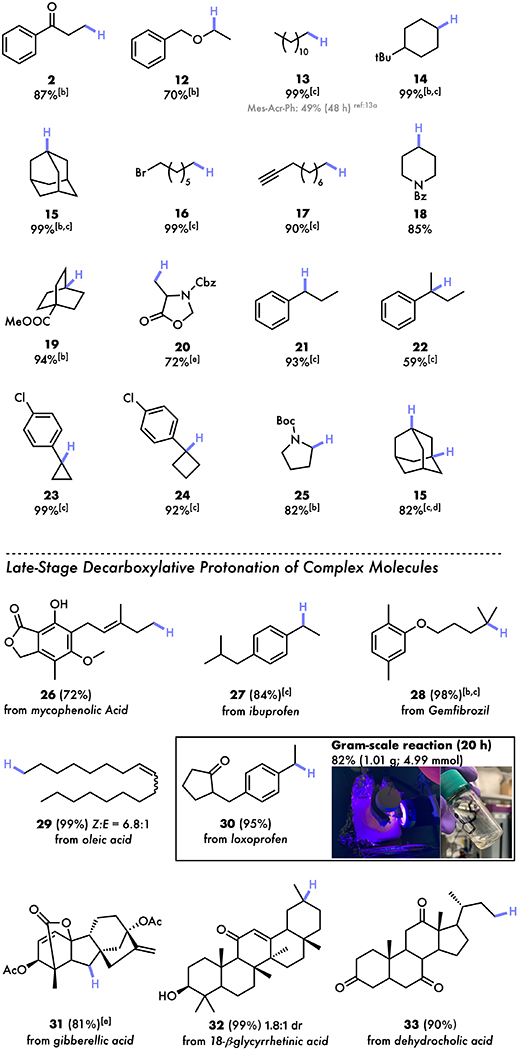
|
All reactions were conducted on a 0.4 mmol scale.
2.5 mol% Fe(NO3)3·9H2O and 2.5 mol% di(2-picolyl)amine were used.
The yields were determined by 1H NMR spectroscopy using trimethoxybenzene as an internal standard due to the volatile properties or poor traceability on thin layer chromatography.
Reaction concentration = 0.05 M; 30 mol% TRIP disulfide were used.
15 mol% TRIP disulfide were used.
