Fig. 3. HCHO reacts with an N-terminal proline to form a methylene bridge between an N-terminal nitrogen and the adjacent peptide bond nitrogen.
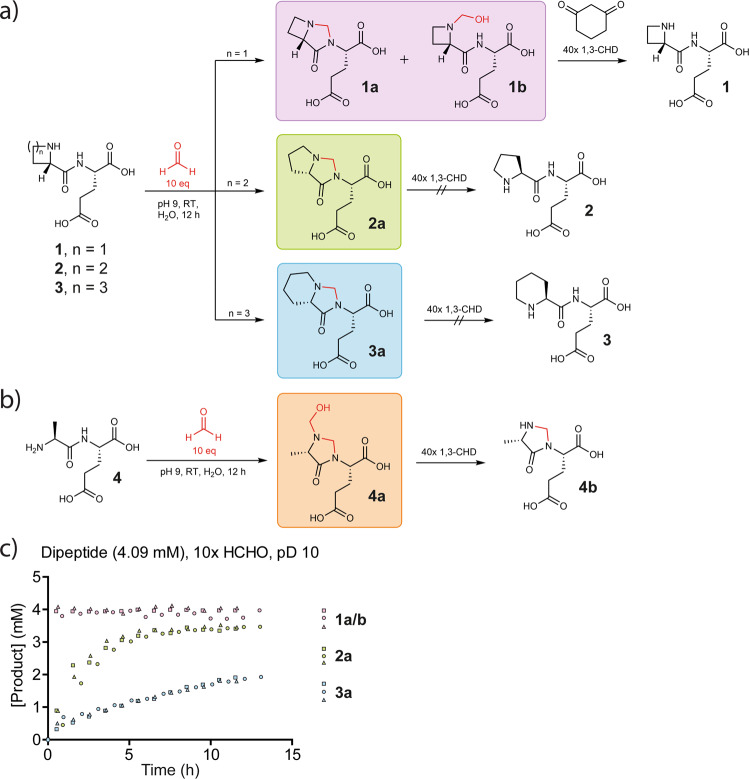
a Products of reactions between HCHO and the dipeptides AzeGlu (n = 1, 1), ProGlu (n = 2, 2), and PipGlu (n = 3, 3). A 10-fold molar excess of aqueous HCHO (410 µmol) was added to each dipeptide (41 µmol). Products containing apparent methylene bridges were observed with all peptides, although the methylene adduct formed with 1 (1a) degraded after addition of the HCHO scavenger 1,3-cyclohexandione (1,3-CHD, 40-fold excess). b AlaGlu (4, 41 µmol) reacts with a 10-fold excess of HCHO (410 µmol) at pD 12 and ambient temperature to form the methylene bridge-containing adduct 4a. Addition of 1,3-CHD (40-fold excess) results in loss of the HCHO-derived hemiaminal from 4a, forming 4b. c Graph showing NMR time-courses monitoring reaction of a 10-fold HCHO excess with dipeptides 1–3 at pD 10 (n = 3 independent replicates); 4 did not react to give an observed product under these conditions (Fig. S18d).