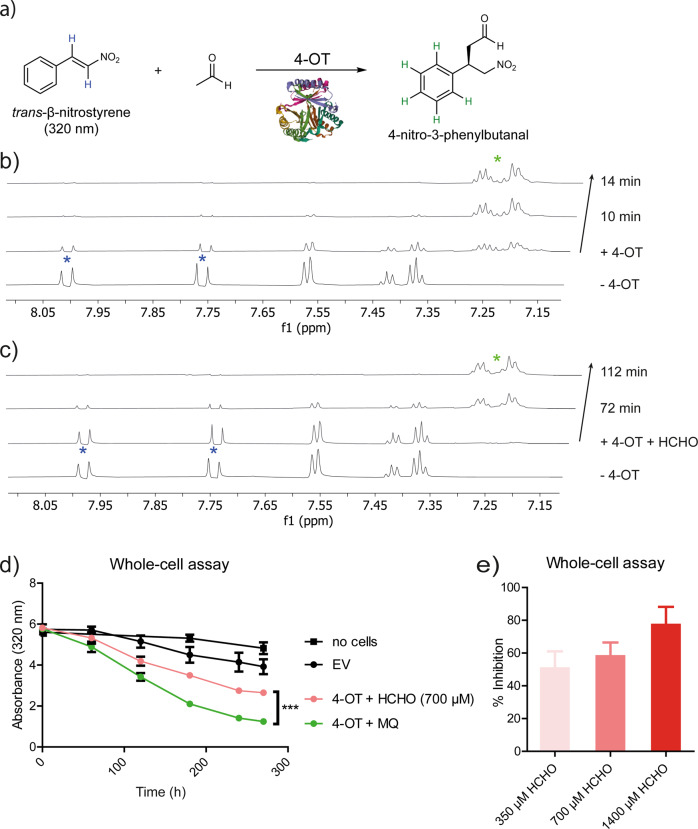
Fig. 4. HCHO inhibits catalysis by isolated 4-OT and 4-OT in cells.
a 4-OT-catalysed reaction of acetaldehyde with trans-β-nitrostyrene to give 4-nitro-3-phenylbutanal. NMR analysis of the alkene hydrogens (blue) and aromatic product hydrogen atoms (green) was used to monitor reaction. Protein image: derived from PDB entry 4X1946. b 1H NMR spectra (700 MHz) showing time-dependent 4-OT-catalysed formation of 4-nitro-3-phenylbutanal (green asterisks). 4-OT was pre-incubated with buffer as a control (17 h, 37 °C). Complete substrate conversion is observed after 14 min. c 1H NMR spectra showing the effect of HCHO on 4-OT-catalysed formation of 4-nitro-3-phenylbutanal. 4-OT (52.9 µM) was pre-incubated with 200 equivalents of HCHO (10.6 mM) for 17 h at 37 °C. Near-complete substrate conversion is only observed after >72 min. Note, at the first analysed time point with HCHO, no product is observed, which implies an IC50 value lower than 10 mM. d Time-dependent depletion of trans-β-nitrostyrene in BL21(DE3) cells transformed with either an empty pET-22b vector (EV), or a pET-22b vector containing the 4-OT gene (4-OT). In cells transformed with the 4-OT vector, trans-β-nitrostyrene depletion is inhibited by addition of HCHO (pink). Green: Milli-Q (MQ) water control of cells transformed with 4-OT. Errors: standard deviation of the mean (n = 3, each replicate in duplicate, statistical analysis: one-way ANOVA); ***p ≤ 0.001. e The same assay as in d was used to determine the extent of inhibition of 4-OT with varied HCHO concentrations; the slope between 60 and 180 min after HCHO addition was used to determine the % inhibition (EC50 ~350 μM). Errors: standard errors of the mean (n = 3, each replicate in duplicate).