FIGURE 6.
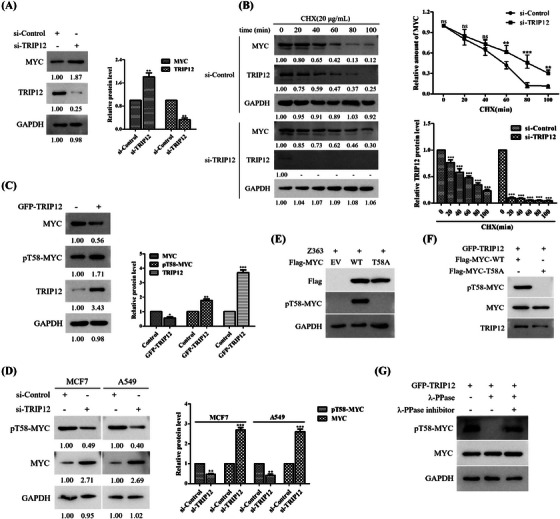
TRIP12 decreases MYC protein stability by modulating MYC‐threonine 58 phosphorylation. (A) MCF7 cells were transfected with Control siRNA or TRIP12 siRNA for 24 h. MYC protein levels were determined by Western blotting. (B) Left panel: MCF7 cells transfected with indicated siRNA for 24 h were treated with CHX (20 μg/ml) for the indicated times and then analysed by Western blotting using indicated antibodies. Right panel: Quantification of the MYC band intensities over time. (C). Overexpression of TRIP12 measured MYC protein level. MCF7 cells were transfected with GFP‐TRIP12, followed by Western blotting with indicated antibodies. (D) MCF7 and A549 cells were transfected with Control or TRIP12 siRNA for 24 h. The expressions of MYC and phosphorylated MYC T58 were analysed by Western blotting. (E) MCF7 cells stably expressing empty vector (EV), wild‐type MYC or MYC T58A mutant were lysed and then analysed by Western blotting using indicated antibodies. (F) Flag‐MYC‐WT or T58A protein were incubated in vitro with immunoprecipitates isolated from MCF7 cells transfected with GFP‐TRIP12 construct and then analysed by Western blotting using indicated antibodies. (G) Lysates prepared from MCF7 cells transfected with GFP‐TRIP12 were treated with λ‐PPase with or without λ‐PPase inhibitor and then analysed by Western blotting as indicated. Data shown in B were analysed by two‐way ANOVA. Blots were representative of three independent experiments. All data are presented as the mean ± SEM of n = 3. ***p < .001, **p < .01, ns, no significance
