FIGURE 7.
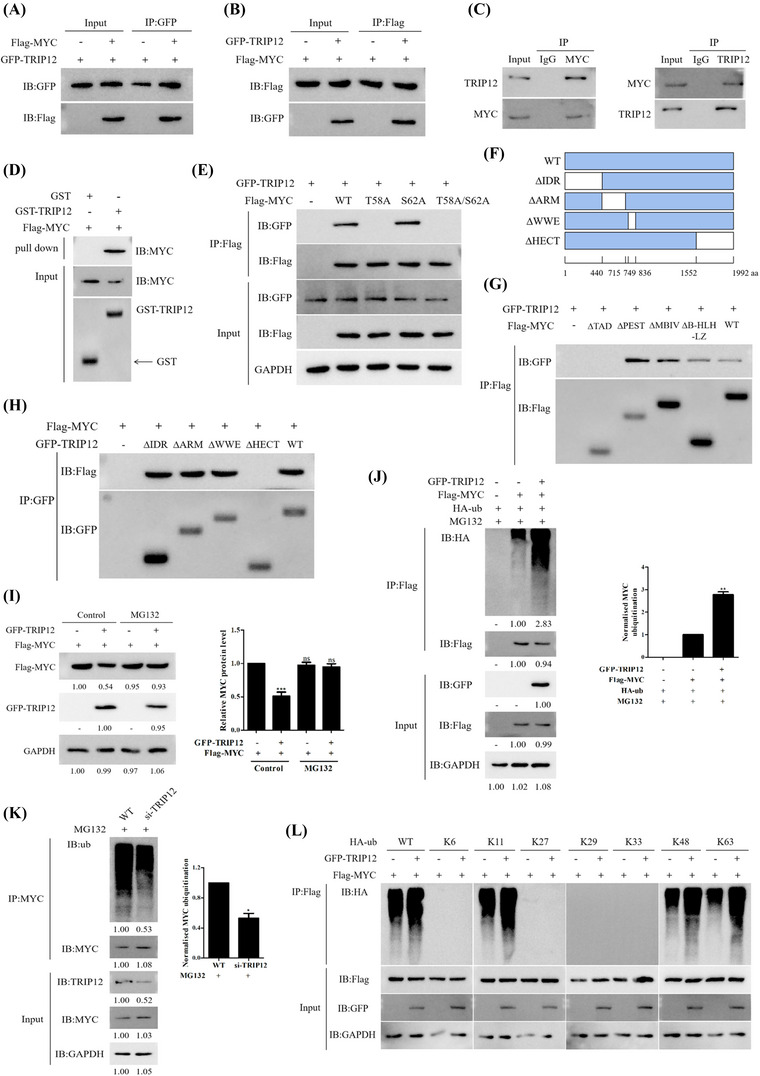
TRIP12 phosphorylation of MYC Thr58 leads to MYC ubiquitination and degradation. (A and B) Flag‐labelled MYC and GFP‐labelled TRIP12 were transfected into MCF7 cells. Cells were treated with Z363 (7.5 μg/ml) for 24 h, and the interaction between MYC and TRIP12 was detected by Co‐IP. (C) Endogenous TRIP12 binds to MYC and vice versa. MCF7 cell lysates were subjected to immunoprecipitation using IgG, anti‐MYC or anti‐TRIP12 antibodies and then analysed by Western blotting as indicated. (D) The interaction between TRIP12 and MYC was detected by GST pulldown assay in vitro. (E) The interaction between the wild‐type MYC as well as its mutants T58A, S62A, T58A/S62A and TRIP12 in MCF7 cells was detected using Co‐IP. (F) Schematic diagram of the TRIP12 mutants. (G) Interaction between the MYC mutants and TRIP12 in MCF7 cells was detected using Co‐IP. (H) Interaction between the TRIP12 mutants and MYC in MCF7 cells was detected using Co‐IP. (I) The proteasome inhibitor MG132 blocks TRIP12‐induced MYC degradation. MCF7 cells were transfected with Flag‐MYC together with either GFP‐TRIP12 or empty vector, MG132 (20 μM) was added to the medium 2 h before protein harvest. (J) HA‐ub, Flag‐MYC, and GFP‐TRIP12 plasmids were transfected alone or co‐transfected into MCF7 cells, and the ubiquitination of MYC was detected by Co‐IP. (K) Ubiquitination of MYC was analysed in WT and si‐TRIP12 MCF7 cells. (L) Lys‐48‐linked ubiquitination catalysed by the wild‐type TRIP12 (GFP‐TRIP12‐WT) was further confirmed by seven Lys‐only ubiquitin mutants, Lys‐6, ‐11, ‐27, ‐29, ‐33, ‐48 and ‐63. Blots were representative of three independent experiments.
