FIGURE 1.
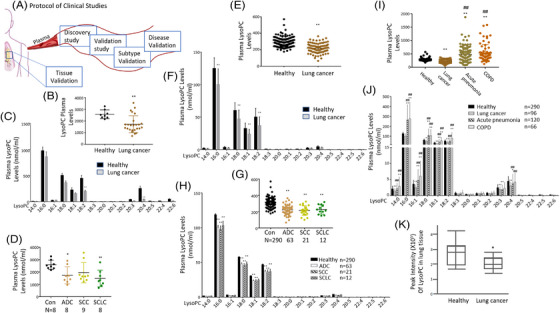
A series of clinical open random studies to evidence lysophosphatidylcholine (lysoPC) alterations. The protocol of clinical discovery and validation studies was designed prospectively (A), to investigate plasma levels of total lysoPC (B) and lysoPC 15 species (C) between lung cancer patients (n = 25) and healthy (n = 8) as well as plasma levels of total lysoPC (D) in lung cancer subtypes (adenocarcinoma [ADC] [n = 8], squamous cell carcinoma [SCC] [n = 9] or small cell lung cancer [SCLC] [n = 8]) in the discovery study. The validation studies included plasma levels of total lysoPC (E) and lysoPC species (F) between healthy (n = 290) and lung cancer patients (n = 96) as the first part; plasma levels of total lysoPC (G) and lysoPC species (H) between healthy and patients with ADC (n = 63), SCC (n = 21) and SCLC (n = 12) as the second part; plasma levels of total lysoPC (I) and lysoPC species (J) between healthy and patients with lung cancer, acute pneumonia (n = 120) or COPD (n = 66) as the third part, as well as lung tissue lysoPC levels (K) between lung cancer tissues and corresponding para‐cancer tissues (n = 10 pairs). * and ** stand for the p‐values less than .05 and .01, respectively, as compared with the healthy
