FIGURE 2.
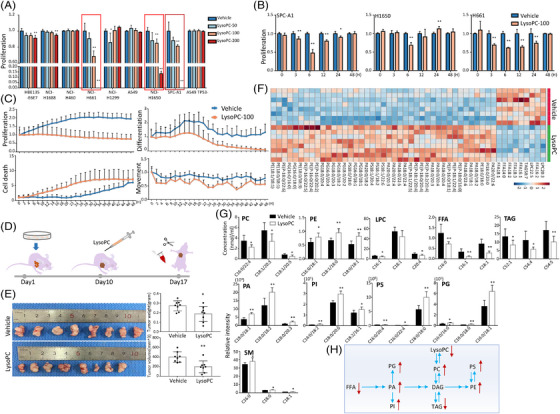
Validation of exogenous lysophosphatidylcholine (lysoPC) effects on lung cancer in preclinical studies, Effects of exogenous lysoPC at different concentrations on the proliferation of nine lung epithelial cells (A; n = 5 per group) was validated, from which the proliferation of SPC‐A1, NCI‐H1650 and NCI‐H661 was assessed after once treatment with lysoPC at the concentration of 100 μM for time points (B) and bio‐behaviours (proliferation, death, differentiation and movement) of SPC‐A1 cells after continuous treatment with lysoPC at 100 μM for 48 h (C; n = 12 per group). The protocol of evaluation on the effects of lysoPC in an animal model was shown in D. Human SPC‐A1 cells were transplanted into the armpits of mice on day 1 and the tumours grew for 10 days. The experimental group was intraperitoneally injected with vehicle or lysoPC on day 10 and the experiment was terminated seven consecutive days after the injection, and the tumour and blood were sampled for further analyses, including mouse tumour weight and size (E), heatmap of top 50 lipid levels with statistical significance (F) and plasma levels of representative lipid species (G) between animals treated with vehicle or lysoPC (n = 8 per group). The metabolic pathway of lipid changes in the blood of an in vivo mouse model was also described (H). * and ** stand for the p‐values less than .05 and .01, respectively, as compared with the groups treated with vehicle
