FIGURE 3.
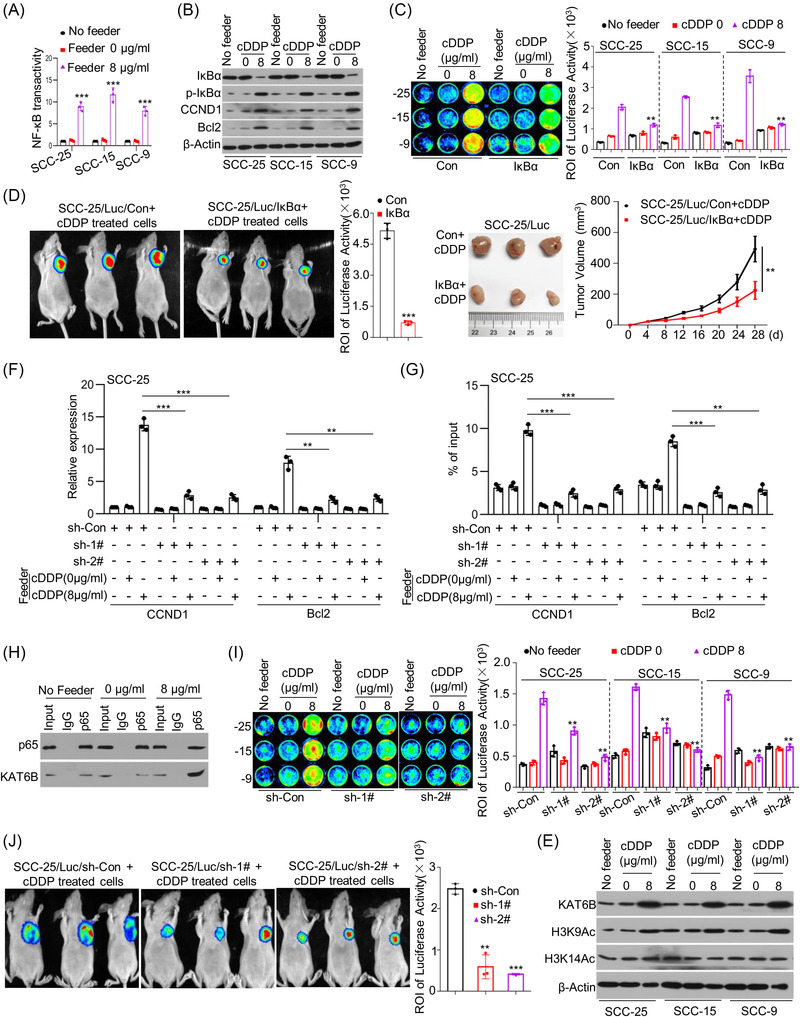
Lysine acetyltransferase 6B (KAT6B)‐dependent nuclear factor‐kappa B (NF‐κB) signalling is responsible for living tumour cell repopulation stimulated by dying cells. (A) Briefly, SCC‐25, SCC‐15 and SCC‐9 cells were treated with cisplatin (cDDP) for 24 h. Then, another SCC‐25, SCC‐15 and SCC‐9 cells were seeded among each cDDP‐treated cells. The transactivation of NF‐κB signalling in cells was measured by Cignal Reporter Assays (n = 3), *** p < .001. (B) SCC‐25, SCC‐15 and SCC‐9 cells were treated with cDDP for 24 h. Then, new SCC‐25, SCC‐15 and SCC‐9 cells were seeded among each cDDP‐treated cells. The protein levels of IκBα, p‐IκBα, CCND1, Bcl2 and β‐Actin in these cells were detected by western blots. (C) SCC‐25, SCC‐15 and SCC‐9 feeder cells were treated with cDDP for 24 h. SCC‐25/Luc, SCC‐15/Luc and SCC‐9/Luc reporter cells were transfected with pBabe‐Con or pBabe‐IκBα, and then seeded among the respective feeder cells with cDDP treatment or alone in 24‐well plates. Cancer cell repopulation in vitro was evaluated by luciferase activities, ** p < .01. (D) SCC‐25 cells were treated with cDDP for 24 h. SCC‐25/Luc reporter cells were transfected with pBabe‐Con or pBabe‐IκBα and injected subcutaneously together with cDDP‐treated SCC‐25 cells into nude mice. The growth of SCC‐25/Luc cells transfected with pBabe‐Con or pBabe‐IκBα was represented by luciferase levels and tumor size, ** p < .01, *** p < .001. (E) SCC‐25, SCC‐15 and SCC‐9 cells were cocultured with cDDP‐treated each feeder cells. The protein levels of KAT6B, H3K9Ac, H3K14Ac and β‐Actin were detected by western blots. (F and G) SCC‐25 cells were treated with cDDP for 24 h, another SCC‐25 cells were transfected with shRNAs specifically targeting KAT6B and then cocultured with cDDP‐treated SCC‐25 cells. The expression levels of CCND1 and Bcl2 were detected by quantitative real‐time polymerase chain reaction (qRT‐PCR) (n = 3) (F). The H3K9Ac levels at the promoter regions of CCND1 and Bcl2 were detected by chromatin immunoprecipitation (ChIP)‐qPCR (n = 3), ** p < .01, *** p < .001 (G). (H) SCC‐25 cells were treated with cDDP for 24 h, another SCC‐25 cells were cocultured with cDDP‐treated SCC‐25 cells. Total proteins were subjected to immunoprecipitation (IP) using an anti‐p65 antibody or control immunoglobulin G (IgG), followed by western blot analysis with a specific antibody against KAT6B. (I) SCC‐25 cells were treated with cDDP for 24 h. SCC‐25/Luc, SCC‐15/Luc and SCC‐9/Luc reporter cells were transfected with shRNAs specifically targeting KAT6B and then seeded among the respective feeder cells with cDDP treatment or alone in 24‐well plates. Cancer cell repopulation in vitro was observed by luciferase activities, ** p < .01. (J) SCC‐25 cells were treated with cDDP for 24 h. SCC‐25/Luc cells with or without KAT6B knockdown were injected subcutaneously together with cDDP‐treated SCC‐25 cells in nude mice, and tumour growth is represented by luciferase levels, ** p < .01, *** p < .001. Data in (A), (C), (D), (F), (G), (I) and (J) are represented as the mean ±SEM. Statistical significance was determined by a two‐tailed Student's t‐test
