Abstract
Consumption of alcohol in excess leads to substantial medical, economic, and societal burdens. Approximately 5.3% of all global deaths may be attributed to alcohol consumption. Moreover, the burden of alcohol associated liver disease (ALD) accounts for 5.1% of all disease and injury worldwide. Alcohol use disorder (AUD) affects men more than women globally with significant years of life loss to disability in low, middle and well-developed countries. Precise data on global estimates of alcohol related steatosis, alcohol related hepatitis, and alcohol related cirrhosis have been challenging to obtain. In the United States (US), alcohol related steatosis has been estimated at 4.3% based on NHANES data which has remained stable over 14 years. However, alcohol-related fibrotic liver disease has increased over the same period. In those with AUD, the prevalence of alcohol related hepatitis has been estimated at 10–35%. Globally, the prevalence of alcohol-associated cirrhosis has been estimated at 23.6 million individuals for compensated cirrhosis and 2.46 million for those with decompensated cirrhosis. The contribution of ALD to global mortality and disease burden of liver related deaths is substantial. In 2016 liver disease related to AUD contributed to 50% of the estimated liver disease deaths for age groups 15 years and above. Data from the US report high cost burdens associated with those admitted with alcohol-related liver complications. Finally, the recent COVID-19 pandemic has been associated with marked increase in alcohol consumption worldwide and will likely increase the burden of ALD.
Keywords: alcoholic steatosis, alcoholic hepatitis, alcoholic cirrhosis, alcohol, alcohol use disorder
Abbreviations: AAPC, Average annual percentage change; ABIC, Age, Serum bilirubin, INR and serum Creatinine; ABV, Alcohol by volume; ALD, Alcohol-associated liver disease; AUD, Alcohol use disorder; BAC, Blood alcohol concentration; CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; GAHS, Glasgow alcoholic hepatitis score; HE, Hepatic encephalopathy; HRS, Hepatorenal syndrome; ICD-10, International Classification of Diseases, 10th Edition; MDF, Maddrey's Discriminant Function; MELD, Model of end-stage liver disease; MRI, Magnetic resonance imaging; NIAAA, National Institute of Alcohol Abuse and Alcoholism; NIS, National inpatient sample; NHANES, National Health and Nutrition Examination Survey; NSDUH, Annual National Survey on Drug Use and Health; SAMHSA, Substance Abuse and Mental Health Services Administration; US, United States; USG, Ultrasonography; WHO, World Health Organization; YLD, Years of life lost to disability
The consumption of alcohol has been prevalent in different cultures for many centuries. Based on a recent analysis in 2014, the cultural influence has remained an essential correlate of alcohol use and misuse for over 75 years.1 Consumption of alcohol in large quantities can lead to substantial medical, economic, and social burdens on societies in terms of morbidity and mortality. According to reports from WHO in 2016, about 5.3% of all global deaths were attributed to alcohol consumption or approximately 3 million deaths on a global scale.2 The global burden of alcohol-associated liver disease accounts for about 5.1% of disease and injury worldwide, is one of the most frequent causes of death in the world, ranking 30th, and is directly related to the amount of alcohol consumption.3 Globally, AUD affects men more than women and is the second most disabling disease and injury condition for men.4 One estimate suggests that AUD may cause 18.4 million years of life lost to disability (YLD) which extrapolates to about 3.5 percent of all YLDs, in low and middle-income countries and 5.7 percent of all YLDs in well-developed countries.4,5 In this review, we will discuss the evolving nomenclature and standard measurements for AUD, as well providing estimates for disease burden across the spectrum of alcohol-associated liver disease and factors that contribute to progression of alcohol-associated liver disease. Finally, we will address the effects of the recent COVID-19 pandemic on the disease burden of ALD.
Definitions associated with AUD
When estimating the epidemiological burden of alcohol associated liver disease, it is important to understand the different definitions used in association with AUD. Binge drinking, heavy drinking, or any drinking by pregnant women or people younger than 21 is considered excessive drinking. According to NIAAA (National Institute of Alcohol Abuse and Alcoholism) guidelines, binge drinking includes four or more drinks for women and five or more drinks for men on a single occasion or in 2 h. This equates to a drinking pattern that can increase blood alcohol concentration (BAC) to 0.08 percent or 0.08 g of alcohol per deciliter or higher.6 In contrast, heavy drinking is defined as the consumption of more than three drinks on any day or more than seven drinks per week for women and four drinks on any day or more than fourteen drinks per week for men4 Other classifications include the Annual National Survey on Drug Use and Health (NSDUH), conducted by the Substance Abuse and Mental Health Services Administration (SAMHSA), which defines binge drinking as five or more alcoholic drinks for males or four or more alcoholic drinks for females on the same occasion (i.e., at the same time or within a couple of hours of each other) on at least one day in the past month. Though immoderate drinking does not necessarily make a person alcohol dependent, the risk of AUD increases significantly with binge drinking and heavy alcohol use.7
The Centers for Disease Control (CDC) has provided guidance for the amount alcohol in a standard drink with a standard drink comprising 12 ounces of 5% alcohol by volume (ABV) for beer, 8 ounces of 7% ABV for malt liquor, 5 ounces of 12% ABV for wine, and 1.5 ounces of 40% ABV distilled spirits or alcohol, including gin, rum, vodka, and whiskey. In addition, the CDC estimates that excessive drinking is responsible for approximately 88,000 deaths per year, with a total economic cost burden of 249 billion per year that excludes costs from motor vehicle accidents, violence, injuries, risky sexual behaviors, and chronic conditions such as cancer, hypertension, and heart diseases.8
As we discuss the disease burden of ALD, it is important to note that more than 30 conditions are associated with alcohol consumption, with alcohol being the primary driver in these cases according to WHO International Classification of Diseases, 10th Edition (ICD–10).9 AUD remains one of the most critical disease illnesses in this group and entails alcohol dependence and alcohol abuse. AUD is associated with considerable disability, being the fourth most disabling disease category in low to middle-income countries and the third most disabling disease category in well-developed countries according to a recent report.10 Based on reports from 2016 National Survey on Drug Use and Health, approximately 136 million US citizens aged 18 and older drink alcohol, with 65.3 million reporting binge drinking in the past month, accounting for half (47%) of the current alcohol users. In addition, 16.3 million also reported heavy drinking in the past month and accounting for 24.9% of individuals who reported binge drinking and 11.9% of individuals with current alcohol use.11 Approximately 35,000 deaths per year are attributable to alcohol-associated cirrhosis, making alcohol-associated liver disease (ALD) one of the two leading indications for liver transplant.11,12
Key points
Different definitions used in association with AUD are important in order to understand the epidemiological burden of alcohol associated liver disease. Approximately, 35,000 deaths per year are attributable to alcohol associated cirrhosis, making ALD as one of the two leading indications for liver transplant.
The Spectrum of Alcohol-associated Liver Diseases
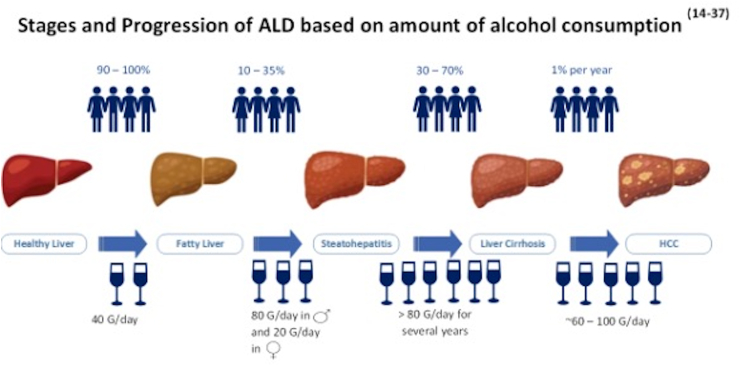
In the Western world, AUD represents one of the most common causes of liver damage. The spectrum of liver diseases associated with AUD ranges from simple steatosis, to steatohepatitis, fibrosis, cirrhosis, and cancer.13 The International Classification of Diseases (ICD-10) recognizes these stages of liver damage, including steatosis, steatohepatitis, and early fibrosis, as being considered reversible, while late stages constituting cirrhosis and liver failure and cancer are considered severe and irreversible (Figure 1).14 The various stages of ALD and severity are directly related to the amount of alcohol consumed.15 Literature suggests that alcohol consumption of more than 80 g/day in men and 20 g/day in women is associated with liver cirrhosis.16 A study from northern Italy examined a total of 6534 subjects, indicating an increased risk of developing either noncirrhotic alcohol-induced liver injury or alcohol-associated cirrhosis in both men and women who consumed at least 30 g of alcohol per day and the risk of ALD substantially increased with increasing alcohol intake.17
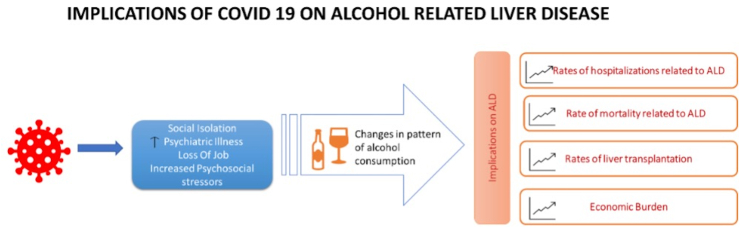
Figure 1.
Implications of COVID 19 on alcohol related liver disease.
Fatty liver (steatosis) is the earliest, most common response of the liver to moderate or large doses (i.e., binge drinking) of alcohol and chronic ethanol consumption. This is attributed to changes in lipid metabolism in the liver with an increase in lipid synthesis and decreased lipid metabolism, causing lipid storage in the hepatocytes. Though fatty liver caused by alcohol consumption was once considered benign, it is now recognized as the leading risk factor for liver disease progression and even cirrhosis.18 Dose and duration of alcohol intake remain the primary drivers of alcohol-related steatosis in this case though the exact amount of alcohol intake required for the development of steatosis remains controversial. Based on a study done by Lieber et al., hepatic steatosis may develop after 2–3 weeks of excessive alcohol intake (120–150 g/dl per day), and if alcohol consumption continues, there may be progression to severe inflammation with alcohol-related hepatitis.19,20
Alcohol-related hepatitis has been associated with high rates of mortality and morbidity, with severe cases having short term (30 day) mortality rates ranging between 30 and 50 percent.21,22 Alcohol-related hepatitis is characterized by a constellation of clinical signs and symptoms including fever, elevated bilirubin levels (>2), coagulopathy, and liver-related complications such as ascites, volume overload, hepatic encephalopathy, and GI bleeding. Several prognostic scores have been used to predict short-term mortality in these patients. These include MDF (Maddrey's Discriminant Function), MELD (Model of End-stage Liver Disease), ABIC (Age, Serum bilirubin, INR and serum Creatinine), GAHS (Glasgow Alcoholic Hepatitis Score), and Lillie model. The role of these scoring systems remains limited to determining the long term survival of these patients, as they depend primarily on abstinence from alcohol.22
Excessive and continued alcohol consumption increases the risk of developing alcohol-related cirrhosis.23 The exact amount of alcohol consumption for the development of alcohol-related hepatitis and its progression to liver cirrhosis is not yet determined. However, based on prior observational studies, ingestion of more than >20 g of alcohol per day in women and >80 g per day in men is associated with an increased prevalence of liver cirrhosis.17 Signs and symptoms of patients with alcohol-related cirrhosis are similar to other chronic liver diseases and can be further characterized into compensated and decompensated liver cirrhosis. Decompensated liver cirrhosis is associated with complications such as ascites, hepatic encephalopathy (HE), GI bleeding, hepatorenal syndrome (HRS), and death.14
Key points
Various stages and severity of ALD is directly related to amount of alcohol consumed. The International Classification of Diseases (ICD-10) recognizes these stages of liver damage, including steatosis, steatohepatitis, and early fibrosis, as being considered reversible, while late stages constituting cirrhosis and liver failure and cancer are considered severe and irreversible.
Incidence and Prevalence of Alcohol Related Steatosis, Alcohol Related Hepatitis, and Alcohol-Related Cirrhosis
Epidemiology of Alcohol-related Steatosis
The prevalence of alcohol related steatosis is challenging to ascertain in the general population as many patients do not seek medical attention due to the asymptomatic nature of the disease.24 The estimated prevalence also depends on the diagnostic modality used to screen for steatosis. Methods used to ascertain steatosis include ultrasonography (USG), magnetic resonance imaging (MRI), and liver biopsy. Amongst these, USG is the least expensive and non -invasive method with a sensitivity of 60–90 percent and specificity of 90–95 percent, making it an acceptable choice in the clinical care setting. Magnetic resonance imaging increases the accuracy of detection of alcohol-related steatosis and can help quantify the degree of hepatic steatosis though it is an expensive modality.25 Liver biopsy remains the gold standard for detection of steatosis; however, its routine use is limited by its invasive nature and related complications, making it a less desirable choice and impractical modality in the clinical setting for steatosis assessment.26
The prevalence of alcohol-related steatosis in the US has been estimated using NHANES data derived from questionnaires, abdominal ultrasonography, and laboratory tests. Based on one study, the prevalence of alcohol related-steatosis was around 4.3 percent in the US population among 34, 423 respondents. There was a stable trend over the last 14 year study period from 2001 to 2016; however alcohol-related steatosis associated with stage 2 fibrosis showed an increasing trend from 0.6 to 1.5 percent.27,28 Though alcohol-related steatosis has been considered a benign condition, it is now known that there may be progression to advanced fibrosis and cirrhosis, leading to increased morbidity and mortality rates in this patient population. A recent meta-analysis estimated the annual rate of progression of alcohol-related steatosis to cirrhosis at approximately 3 percent, with mortality rate of 6 percent. Interestingly, these patients who progress also tend to have increased mortality from non-hepatic causes when compared with liver-specific mortality rates.29
Another retrospective study using NHANES data analyzed and compared the prevalence of ALD also over the period of 2001–2016. In this study the overall weighted prevalence of ALD remained stable at 8.8% (95% CI: 7.6–9.9) and 8% (95% CI: 6.5–9.6) in the year 2001–2002 and 2015–2016 respectively (P = 0.102). However, there was an increase in the prevalence of Stage 3 fibrosis in ALD patients from 2.2% (95% CI: 0.4–4.0) to 6.6% (95% CI: 2.0–9.9) over the same period (P = 0.007). Based on the same survey study, the estimated national prevalence of ALD and ALD with stage 3 fibrosis was calculated to be 17.8 million (95% CI: 14.3–21.3) and 1.1 million (95% CI: 0.4–1.8) in the year 2015–2016. The sex specific trends remained stable over the study period, though there was higher prevalence of ALD in men compared to women.27 (Table 1).
Key points
Based on NHANES data from 2001 to 2016, alcohol related steatosis has been stable over the last 14 year study period, while stage 2 fibrosis associated with alcohol related steatosis showed an increasing trend from 0.6 to 1.5 percent.
Table 1.
Estimated Prevalence of Alcoholic LiverDisease (ALD) in USUsing NHANES DataComparingYears 2001–2002 and 2015-2016.29
| Alcoholic Liver Disease (ALD) | Year: 2001–2002 | Year: 2015–2016 |
|---|---|---|
| Overall weighted Prevalence of ALD | 8.8% (95% CI: 7.6–9.9) | 8.1% (95% CI: 6.5–9.6) |
| ALD with Stage ≥3 Fibrosis | 2.2% (95% CI: 0.4–4.0) | 6.6% (95% CI: 2.0–9.9) |
| Prevalence of ALD in Men | 9.7% (95% CI: 7.9–11.4) | 9.0% (95% CI: 6.2–11.7) |
| Prevalence of ALD in Women | 8.0% (95% CI: 6.3–9.7) | 7.2% (95% CI: 5.9–8.5) |
| Prevalence of ALD with Stage ≥3 Fibrosis in Men | 2.4% (95% CI: 0.01–9.0) | 7.3% (95% CI: 0.01–15.5) |
| Prevalence of ALD with Stage ≥3 fibrosis in Women | 2.6% (95% CI: 0.4–4.8) | 4.6% (95% CI: 0.01–9.2) |
Abbreviation: ALD, Alcohol-associated liver disease.
Epidemiology of Alcohol-related Hepatitis
The precise incidence and prevalence of alcohol-related hepatitis are not known with one of the main challenges being asymptomatic patients often remain undiagnosed. According to a study conducted by Naveau et al., the prevalence of alcohol related hepatitis was approximately 20% in a cohort of 1604 patients with AUD with available liver biopsies. Approximately 10–35% of individuals with AUD will have changes consistent with alcohol related hepatitis and an estimate of the prevalence of alcohol-related hepatitis can be extrapolated based on the prevalence of AUD. In the US, the prevalence of AUD has been estimated at 16 million people, or 8% of the general population; hence the number of individuals with AH in the US may approximate up to 5 million individuals.30
The National Inpatient Sample (NIS) Database also provides insights into the epidemiology of AH in the US. Based on inpatient data an increase in AH-related hospitalizations has been reported from 2002 (249,884 or 0.66% of total admission in 2002) to 2010 (326,403 or 0.83% of total admissions). There was also an increased rate of readmissions in this patient population given the significant co-morbidities and complications including increased rates of sepsis, acute renal insufficiency, and gastrointestinal bleeding31 (Table 2). Reported readmission rates in these patients range from 20 to 25% at 30 days to 37% at 90 days after discharge. Based on data from National Readmission Database involving 21,572 individuals with alcohol-related hepatitis, the annual cost burden at 30 day and 90 day for AH-related readmissions was approximated at $164 million for 30 day readmissions and $321 million for 90 day readmissions respectively in this cohort.32 In addition, rates of rehospitalizations based on an analysis of over 15,000 commercially insured adults with AH demonstrated more than 50% of survivors were hospitalized within one year and 75% through the second year with total number of readmissions reaching as high as 40,000. The total health care costs were approximated at $2.2 billion over a 5 year period including costs of care associated with liver transplantation.33 Based on a recent systematic review of 7528 participants, the annual rate of progression of AH to cirrhosis was estimated at 10% with a similar rate of progression to cirrhosis of 8% noted in those with fibrotic liver disease. In addition, there is a significant increase in mortality in patients with alcohol related hepatitis ranging from 5 to 15% annually with the highest rates noted in those with alcohol related hepatitis who required inpatient level of care29 (see Table 3).
Key points
Based on NIS data base analysis, there has been an increase in the rate of readmissions in patients with alcohol related hepatitis due to higher rates of complications such as sepsis, acute renal insufficiency, and gastrointestinal bleeding in this patient population. In addition, there is a significant increase in mortality in patients with alcohol related hepatitis ranging from 5 to 15% annually with the highest rates noted in those with alcohol related hepatitis who required inpatient level of care.
Table 2.
Trends in Alcoholic Hepatitis Related Hospitalizations, Financial Burden and Mortality Rates in USA Using NIS Data Base Analysis (2002–2010).33
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|
| Alcohlic hepatitis related hospitalizations | 249,884 (0.66) | 249,437 (0.65) | 265,072 (0.69) | 265,174 (0.68) | 286,160 (0.73) | 286,018 (0.72) | 301,197 (0.76) | 310,745 (0.79) | 326,403 (0.83) |
| Overall health care cost ($) | 25,276 ± 390 | 30,049 ± 500 | 28,639 ± 400 | 32,077 ± 470 | 34,033 ± 446 | 36,601 ± 520 | 37,820 ± 514 | 39,998 ± 524 | 40,870 ± 488 |
| Mortality (%) | 8 | 8.1 | 7.5 | 7.5 | 7.1 | 6.5 | 5.9 | 5.6 | 5.1 |
Abbreviation: NIS, National inpatient sample.
Table 3.
Preliminary Reports on Effect of COVID Pandemic on Drinking Patterns Worldwide.
| Author | Population | N | Findings |
|---|---|---|---|
| Grossman et al.47 | US adults above 21 yrs. | 832 | Increase in binge drinking and frequency of alcohol use |
| Chartier et al.52 | US adults 18 yrs and older | 5874 | Increased frequency of alcohol use during 3rd wave of COVID-19 amongst users of multiple social media platforms |
| Zipursky et al.53 | Canadian study | NA | Increase in alcohol sales and alcohol related emergencies |
| Sidor et al.54 | Polish residents | 1097 | Increase in alcohol consumption was seen in 14.6%, with a higher tendency to drink more in individuals with AUD |
| Kar et al.55 | Pregnant individuals in Canada | 7470 | 6.7% of pregnant females reported increased alcohol use |
| Boschuetz et al.56 | US adults | 417 | Increase in AUDIT C score in females, increase in the frequency and quantity of alcohol use as well as the frequency of alcohol use prior to 5 pm. |
| Hanson et al.57 | American Indian women | 62 | Increase in drinking amongst binge drinkers by 24.2%, over half had at least one binge drinking episode. Approximately half reported reduced drinking. |
| Vandenberg et al.60 | Australian residents >15 yrs old | N/A | Reduction in on premises beer per capita consumption during first wave restrictions of COVID pandemic while partial removal of restrictions resulted in immediate increase in on premise beer per capita consumption |
| Naughton et al.61 | UK residents 18 yrs or older | 1044 | Increased alcohol intake (AUDIT C scores) during first wave of COVID pandemic |
| Bollen et al.62 | French speaking Belgian adults | 1693 | Decreased their alcohol consumption after lockdown onset and returned to their initial alcohol consumption after lockdown offset |
| Hagit Bonny Noach63 | Israeli adult residents age 18-65 | 750 | Participants who experienced two lockdowns reported more frequent consumption of all alcoholic beverages and cannabis in the last 30 days than those who experienced one lockdown |
| Szajnoga et al.65 | Polish adults | 4072 | Increased alcohol use amongst particular groups including men, 18–24 yrs of age, inhabitants of big cities, and remote workers |
| Sallie et al.66 | Multinational adults 18yrs or older | 2873 | 36% adults reported an increase in alcohol use during COVID-19 pandemic |
| Mangot Sala et al.67 | Netherland | 63,194 | Decreased alcohol consumption during COVID pandemic compared to previous years |
| Bonar et al.68 | US University students | 741 | 39.4% reported lower binge drinking post campus closure while 4.18% reported higher 30 day binge drinking frequency post campus closure |
| Hooijdonk et al.69 | Dutch University students | 9967 | Decrease in weekly binge drinking |
| Valente et al.70 | Latin American and Caribbean adults | 12,328 | Increase in heavy episodic drinking amongst individuals with AUD |
| Morales et al.71 | Mexican adults | 9361 | Poor mental health was found a predictor of increased alcohol use during COVID-19 pandemic |
| Weerakoon et al.72 | US adults | 1982 | 34% of the sample reported increased binge drinking. Increased binge drinking was seen among binge drinkers compared to non-binge drinkers |
| Vasconcelos et al.73 | Portuguese college students | 146 | Decreased alcohol consumption in binge drinkers during lockdown |
Abbreviations: AUD, Alcohol use disorder; AUDIT C, AUD Identification Test.
Epidemiology of Alcohol-associated Cirrhosis
According to a systematic analysis for the global burden of disease study in 2017, the global prevalence of ALD has been estimated to be around 23.6 million and 2.46 million for both compensated and decompensated cirrhosis respectively. There has been an increasing trend towards age-standardized prevalence of patients with decompensated cirrhosis from all etiologies that has occurred from 1990 to 2017 with prevalence increasing from 25.3 per 100,000 in 1990 to 30 per 100,000 in 2017. On the other hand, the trend for compensated cirrhosis has been relatively stable with 290 per 100,000 in 1990 to 288.1 per 100,000 in 2017.34 Based on reports from NHANES survey from 1988 to 1994 and 1999–2016, the total prevalence of ALD has been relatively stable in the US from 1988 to 2016.35 However, as noted previously, patients with advanced fibrosis in stages 3 and 4 increased significantly from 2.2 percent in 2002 to 6.6 percent in 2015 and 2016.27 This has been reflected in an estimated increase in rates of hospitalization in patients with alcohol-associated cirrhosis with retrospective analyses in the US noting a 32.8 percent increase in liver cirrhosis related to AUD per 1000 patients with mortality rates as high as 8 percent.27,29 Another observational study done in 2017 estimated the highest average annual percentage change (AAPC) in mortality from cirrhosis of any cause in patients aged 25–34 from year 2009–2016 was 10.5% with CI of 8.9%–12.2% around AAPC.36 Thus, it is not surprising that an 63.4% increase in the listing of these patients for liver transplants was observed during this period.27
A recent study based on NIS database analysis from 2007 to 2014 evaluated annual trends of hospitalizations in patients with alcohol-associated cirrhosis and further stratified it based on age, race and sex yielding useful information about predictors of mortality in these patients. Among 159, 973 hospitalizations with ALD, 83.7% (133, 929) were identified as patients with alcohol-associated cirrhosis based on primary diagnosis. Most of these patients were males (71.45%), and non -Hispanic whites (59.80%) with 29% above 60 years of age. Higher incidence rates of hospitalizations were observed in males compared with females (IRR = 2.68, 95% CI: 2.21–3.71, P < 0.01), those between age 50–59 and 60–69 compared to over 70 years and among Native Americans and Hispanics. Another notable finding was a significant increase in the rates of hospitalization by approximately 20% in patients with alcohol associated cirrhosis from 2007 to 2014. Further stratification of the results showed this increase to be greater in females compared to males (33.5% increase in females vs. 14.7% increase in males, P < 0.01), and among Native Americans and Non-Hispanic whites compared to African Americans, Asian/Pacific Islanders and Hispanic patients. Mortality data from the same study demonstrated higher mortality rates among patients hospitalized with alcohol-associated cirrhosis compared to alcohol related hepatitis (6.98% compared to 2.97% respectively). Among patients with alcohol associated cirrhosis, higher mortality rates were observed in non-Hispanic whites compared to Hispanics (7.25% vs 5.95%, P < 0.001), with African Americans showing significantly higher odds of mortality on multivariate regression analysis ((1.13, 95% CI: 1.04–1.24, P < 0.01]). In addition, patients who were self-pay/uninsured showed higher in hospital mortality (OR = 1.506, 95% CI: 1.39–1.64, P < 0.001), in comparison to Medicare patient population (OR = 0.812, 95% CI: 0.75–0.87, P < 0.001). These differences in outcomes should be the focus of future studies to lessen these disparities and allow timely intervention to decrease mortality and morbidity in those with alcohol-associated cirrhosis.37
Key points
Based on observational studies, there has been a notable increase in the rates of hospitalizations in patients with alcohol associated cirrhosis with higher mortality rates seen in these patients compared to alcohol related hepatitis. Mortality rates have been particularly high in African American and non-Hispanic whites and those were self-pay/uninsured.
Global and economic burden of ALD
Global mortality and burden of alcohol-related liver disease are described as disability-adjusted life years. In 2016, chronic liver disease secondary to AUD resulted in 50% of the estimated 1,254,000 liver disease deaths ((588,100; 95% CI: 531,700–683,400; 46.9% of all liver disease, 95% CI: 42.4–54.5) for age groups of 15 years and above with men having a significantly higher number of deaths (416,700, 95% CI: 379,900–514,800) than women (171,400, 95% CI: 134,500–189,700). Based on the on disability-adjusted life years from 2016, 21.5 million years of life were lost due to ALD (21, 476, 000 DALYs; 95% CI: 19,448,000–24,811,000) with men again affected significantly more than women (men: 15, 568, 000 DALYs; 95% CI: 14, 230, 000–19,125,000; women: 5,909,000 DALYs; 95% CI: 4,653,000–6,423,000) with a major portion of these years of lives lost attributable to premature deaths rather than disability 38 In a health care claim analysis report, over 15,000 commercially insured adults with alcohol related hepatitis-related hospitalization from 2006 to 2013 were followed, with 2/3 of the adults dying over five years, and less than 500 receiving liver transplantation. Among those who survived, over 50% were hospitalized within one year and nearly 75% in the second year with total cost expenditure being estimated at $145,000 per patient. This cost burden decreased from about $50,000 in the first year to about $10,000 later. For those who underwent a liver transplant, total transplant-related costs averaged about $300,000, with total health care costs reaching up to $1,000,000 over five years and over five years, the total cost of care for the entire cohort was $2.2 billion.33
Other disparities in the US have been reported. Mortality rates for white women rose from the year 1990–2014, with heavy drinking as one of the major determinants with deaths from cirrhosis almost doubling amongst rural women in their early 50s by the end of the 20th century.39 Another report from the National Academy of Sciences showed a rise in mortality among white non-Hispanic men with AUD related liver cirrhosis as the leading cause of mortality in these patients.40
Key points
Based on the on disability-adjusted life years from 2016, 21.5 million years of life were lost due to ALD with men again affected significantly more than women with a major portion of these years of lives lost attributable to premature deaths rather than disability. Based on health care claim analysis report, those who survived, over 50% were hospitalized within one year and nearly 75% in the second year with total cost expenditure being estimated at $145,000 per patient.
COVID-19 and ALD
Patients with alcohol-associated liver disease have been affected by the COVID-19 pandemic both directly and indirectly (Figure 2). Before the COVID-19 pandemic, national data had shown an increasing trend in drinking and hence overall burden of AUD as previously discussed. This has culminated in an increase in the rates of hospitalizations, liver transplantation, cost, and mortality of patients with alcohol-associated liver disease.41 As previously noted, higher mortality rates in women and younger adults have been observed prior to the pandemic with national death certificate data suggesting an average annual percentage change (AAPC) in mortality rate in women aged 25–34 years of 6.07 (2.95–9.28) compared to women age 65–74 years with AAPC 1.81 (95% CI 0.92–2.71).42 Prior to COVID-19 pandemic, rates of pharmacotherapy and AUD counseling were estimated at 10–14% in patients with alcohol-associated cirrhosis, which indirectly correlates with higher rates of decompensation, mortality, readmission, and relapses in this patient population.42,43
Figure 2.
Stages and progression based on amount of alcohol consumption.
The COVID-19 pandemic has resulted in a drastic increase in alcohol consumption worldwide.44 Based on epidemiological data from US, alcohol use significantly increased in an early pandemic in the general population.45 This surge in alcohol use directly correlated with the increase in online alcohol sales, which showed a spike to 477% in the US compared to previous years46 Households with younger adults, young individuals, and ethnic minorities have shown increasing trends in alcohol purchase during and post COVID pandemic.47 Data from Canada suggests a 38% increase in alcohol purchase during the COVID pandemic, mostly attributed to online sales in the year 2020 compared to 2019. The online purchase of alcohol significantly increased during this time period, given the lower cost of alcohol per unit and this trend is perilous for individuals with AUD.42,48 Based on a telephone survey in the UK, 189 individuals with AUD were queried about their alcohol use during the COVID pandemic with 24% reporting an increase in alcohol use, while only 19% reporting a decrease in alcohol consumption.49 Another finding during the COVID pandemic has identified women with increasing rates of alcohol use which were already accelerating prior to the pandemic. The probable explanation is related to increased workload, including family and child care responsibilities during mandated lockdowns, accompanied by increased work-related responsibilities due to increasing economic burden on women compared to men.50
Key points
The COVID-19 pandemic has resulted in a drastic increase in alcohol consumption worldwide. This culminated in an increase in the rates of hospitalizations, liver transplantation, cost, and mortality of patients with alcohol-associated liver disease.
Alcohol Use in Women During COVID-19
Alcohol use in women has been on the rise during the COVID pandemic with multiple preliminary studies reporting increases in alcohol consumption in females. National surveys conducted before pandemic suggested an increase in AUD (AUD) in women. According to one study, 32% of women aged 15 and older consume alcohol worldwide. Another study reported an estimated increase of 6% in women aged 18 years and older who drank alcohol and about 14% whom binge drank.51 The COVID-19 pandemic has not changed this trend. Preliminary results from the COLLATE project from Australia demonstrated an increased risk of drinking among women during the COVID-19 pandemic.52 A small study conducted in Midwestern females in the US also confirmed an overall increase in alcohol use in women by 17%, and demonstrated an increase in AUDIT C (AUD Identification Test) scores. This increase was seen in both frequency and amount of alcohol consumed.53 Another study reported about 34% of female participants binge drank in the previous month during the early days of the pandemic.47 Additionally, an online survey of over 1000 young female adults in Poland found that 14.6% reported increased alcohol consumption in quarantine during the first wave of COVID-19 pandemic from April–May 2020.54 Based on a survey study from Canada, the most common substance abused by pregnant females during the COVID-19 pandemic was alcohol, followed by Cannabis use, tobacco use, and illicit drugs. However, alcohol and substance use rates were comparable or lower than pre-COVID times in this population.55
Studies have demonstrated various risk factors for an increased risk of drinking among women during the COVID-19 pandemic. Based on a survey study of 417 participants with 83% female representation, a significant increase in the AUDIT C score from 3 to 4 was observed. In this study, 77% of females were married, and 44% were between the ages of 35 and 45. Significant determinants of increased AUD included having children at home, homeschooling, the closing of daycare centers, prior substance use disorder, and pressure for parental involvement56 Jessica et al. investigated alcohol use in pregnant Alaskan Indian/Alaskan native females during the COVID-19 pandemic who were binge drinking prior to the pandemic. The study findings noted the persistence of binge drinking in these female participants, with those who reported less drinking being unmarried or with children sleeping in their rooms noted as protective factors57 A significant increase in wait list additions and transplants for alcohol related hepatitis has been observed in preliminary reports, though gender-based rates of alcohol related hepatitis, listing, and liver transplants, and listing has not changed significantly during the COVID-19 pandemic compared to the pre-pandemic era.58,59
Key points
Alcohol use in women has been on the rise during the COVID pandemic with multiple preliminary studies reporting increases in alcohol consumption in females. This correlates with the reports of increase alcohol use in women before pandemic. Significant determinants of increased AUD during COVID-19 pandemic included having children at home, homeschooling, the closing of daycare centers, prior substance use disorder, and pressure for parental involvement.
Patterns of alcohol use during different waves of COVID-19 pandemic
Multiple studies have reported on the use of alcohol during different waves of the COVID-19 pandemic (Table 3). In another study conducted in the UK during the first wave of the COVID-19 pandemic, there was an increase in alcohol consumption compared to other drugs and an increase in AUDIT C (AUDs Identification Test) scores was observed in these patients due to an increased frequency of alcohol use and not an increase in the number of drinks consumed. This increase in frequency of alcohol use was primarily seen in women and those with increased mental stress levels.60 Another Belgian longitudinal study demonstrated differences in the use of alcohol intake during the COVID pandemic and various stages of lockdown. In their report, the onset of the lockdown was associated with a decrease in alcohol intake, while offsetting lockdown restrictions increased alcohol consumption. Younger individuals and those with AUD were affected by these changes. Protective factors for a reduction in alcohol use included fewer social gatherings, closure of restaurants, and increased awareness regarding alcohol use during the COVID-19 pandemic.61 In another prospective study done in France during the third wave of the COVID-19 pandemic, a significant impact was seen on patients' drinking behavior with AUD with 50% of the patients reporting a significant impact of the COVID-19 pandemic on their drinking behavior. This was reflected in increased short-term drinking in these patients and increased psychological stress. The most critical risk factor associated with an increased risk of alcohol use was craving by multivariate analysis. An Israeli study demonstrated increased alcohol use in patients who experienced two lockdowns compared to those who experienced one lockdown. Interestingly, this pattern of increased alcohol use was not seen with any other drugs of abuse during the COVID-19 pandemic. One explanation for increasing alcohol use may be related to reporting bias as it is legal to buy alcohol in Israel compared to other drugs of abuse.62 In another study by Killgore et al., a total of 5931 adults completed the AUDIT at one of the 6 time points from April through September 2020. Results demonstrated significant increase in heavy alcohol use over the period of 6 months with an upward trend in monthly alcohol use amongst those under COVID lockdown restrictions compared to those who were not under COVID-19 restrictions.63
Data from Australia have conflicting results on alcohol use during the COVID-19 pandemic with some studies reporting an increase in alcohol consumption, while others suggesting a potential decrease in consumption. An interrupted time series analysis performed in Australia suggested varying trends of alcohol use during the lockdown. Based on the data during the first and second wave, there was a decrease in premise-based alcohol consumption, while off-premises alcohol use remained unchanged. However, the removal of sheltering restrictions resulted in an abrupt increase in premise alcohol use. This variation in alcohol use mainly resulted from the closure of restaurants and bars while online alcohol purchase and at-home alcohol use remained unchanged.64 One study noted an overall increase in the use of consumer credit cards by 20% in July 2020 compared to 2019, which was correlated with an increase in off-brand alcohol use in the early pandemic. Increasing alcohol purchase and online delivery sales of alcohol could reflect non-regulation of online purchases, increase in psychological distress, and mental stress during a pandemic. In contrast, another study demonstrated a potential decrease in alcohol use during the COVID-19 pandemic that might have resulted from the economic effects of the pandemic such as loss of job, decreased income, and psychological distress accumulation over time.65 A Polish survey of 4072 individuals suggested an overall decrease in alcohol consumption during the pandemic compared to the pre-pandemic period. Subgroup analysis of the results from the study demonstrated an increase in the frequency of alcohol use in men, inhabitants of large cities, and remote workers.66 An international study involving individuals from 83 different countries was conducted via an online survey to assess changes in drinking behavior before and during COVID-19 lockdown. While the study demonstrated an overall decrease in drinking behavior during the lockdown as demonstrated in some prior studies 36% of patients reported increased alcohol use including essential workers, individuals with children, those with relatives with COVID-19, depression, anxiety, and impulsivity.67 A final study done from the Netherlands demonstrated an overall decrease in the weekly binge drinking patterns in Dutch university students, while cannabis use increased, and weekly smoking remained stable during the COVID-19 pandemic.68
Key points
Social isolation and increased psychological stress has been important determinants of short term increase in alcohol use during COVID-19 pandemic. Decrease in premise-based alcohol consumption was seen during the first and second wave of COVID-19 pandemic, however off premises alcohol use remained largely unchanged.
Binge drinking during the COVID-19 pandemic
Data on college students, teenagers, and adults in different countries have provided insights on binge drinking during COVID-19 pandemic. In a study done on 741 first-year college students, binge drinking was reported in about 6.75% of the students while 39.41% reported decreased episodes of binge drinking after closure of the campuses. Those who reported lower binge drinking showed differences in coping, isolation, and underlying drinking motives, while those who reported more significant binge drinking were in fraternities/sororities and considered this level of drinking a norm.69 Based on data from Dutch university students, weekly binge drinking decreased in students from 27.8% to 13.9% while smoking and cannabis use remained stable.70 A cross-sectional online survey study involving 12,328 adults in 33 countries of Latin America and the Caribbean from Pan-American Health Organization data reported that 65% of drinkers from 2019 reported heavy alcohol drinking during the COVID-19 pandemic with 13.8% reported an increase in heavy alcohol drinking and 33.38% showing a decrease. Factors associated with increased heavy drinking during the pandemic included male gender, higher quarantine practice, and higher income status and generalized anxiety whereas living with children, being a student, and not being employed was associated with the decreased frequency of heavy alcohol use.71 Similar to this report, data from a cross-sectional online survey from Mexico reported poor mental health as one of the factors leading to binge drinking and increased substance abuse.72
Multiple online surveys have been reported from the US. One online survey included 832 respondents and demonstrated an estimated increase in binge drinking of 34.1% of participants, with 7% reporting extreme binge drinking. Patients who reported increased drinking also reported increased stress levels, increased alcohol availability, and boredom47 In another self-reported survey from the US early in the pandemic, included 1982 participants from mid-March to mid-April 2020 with 34% of participants reporting binge drinking. There was an increased alcohol consumption seen in binge drinkers compared to non-binge drinkers with an association of increased time spent at home during the pandemic correlating with binge drinking (OR 1.19 (1.06–1.34). Depressive symptoms were also associated with increased alcohol.73 In a study on Portuguese college students, it was reported that increased alcohol cravings and living with friends were crucial factors of alcohol use during the lockdown. Contrary to other studies, stress anxiety, and depression were not associated with the changes in drinking behavior.74
Key points
There has been mixed reports on binge drinking episodes in college students and teenagers during COVID-19 pandemic. Factors associated with heavy, or binge drinking included male gender, higher quarantine practice, psychiatric illness like anxiety or depression and higher income status.
Drinking Patterns and Correlation Between COVID-19 Pandemic and Great Recession in 2008
An interesting correlation of current data on alcohol use during the COVID-19 pandemic can be made to drinking patterns during the Great Recession in 2008. Though there was an overall decrease in the prevalence of alcohol use or any alcohol drinking during this time, there was a significant increase in binge drinking, which was statistically and epidemiologically significant with risk factors contributing to increased binge drinking including non-Black race, unmarried men under 30, and recent unemployment. Similar trends in alcohol use have been noted during COVID-19 pandemic. This polarization in drinking behavior stemmed from the fact that not all populations are equally affected. Two hypotheses have been proposed to explain the patterns of drinking and include the income hypothesis and provocation hypothesis. The income hypothesis implies that decreased alcohol consumption correlates with a decrease in income affecting mostly young and those with less education leading to less spending on alcohol and hence abstention. The provocation hypothesis is based on an increase in heavy or binge drinking episodes in those with job insecurity, unemployment, and threat of loss of life savings or home.75
Mortality Rates in Patients With AUD During COVID-19
COVID-19 infection has caused increased mortality in patients with AUD due to changes in immune response and severity of the acute respiratory syndrome with multiple factors contributing to the worse outcomes. Individuals with ALD have disrupted innate and acquired immune responses given chronic alcohol use which may result in increased severity of COVID-19 infection.76 ALD patients also have decreased antioxidants in the lungs and changes in alveolar epithelial cells in addition to other comorbidities, including metabolic syndrome, chronic kidney disease, and tobacco use that are associated with worse COVID-19 outcomes. With regard to pre-existing liver disease, multicenter international registries have reported that those with decompensated alcohol cirrhosis, hepatocellular carcinoma, and ALD have worse outcomes with increased morbidity and mortality.77,78
Impact of COVID-19 Pandemic on Liver Transplantation in Alcohol-associated Liver Disease
The full impact of COVID on long-term alcohol intake patterns (rather than short-term changes) on 5-year and 10-year alcohol liver disease burden remains to be defined.79 There are preliminary studies that have investigated the impact of COVID-19 on liver transplantation listing and liver transplantation. A retrospective cohort study using the UNOS database compared listing and rates of liver transplantation in alcohol-associated liver disease during the early pandemic to the pre-pandemic era. A total of 606 candidates with acute alcohol-associated liver disease were identified amongst 38,217 patients on the liver transplant waiting list from March 1, 2018, to February 28, 2021. A mean increase of 106.6% in rate of addition to the waiting list with 210.2% increase in receipt of liver transplants (P < 0.001) compared to the pre-pandemic era.58 Another study examined temporal trends of LT during the COVID-19 pandemic, and demonstrated a significant increase in the rates of liver transplant listing and liver transplant related to ALD (P < 0.01) and was accompanied by an overall decrease in liver transplant listing for hepatitis C -related cirrhosis and nonalcoholic steatohepatitis though the total number of listings and transplants remained unchanged. Not surprisingly young adults demonstrated the greatest increase in ALD, accounting for 33%, with severe ALD (alcohol related hepatitis) accounting for about ∼50% of transplants and high acuity of illness with 40% having MELD score >3059 The increase in alcohol related transplants during the COVID-19 pandemic only reflects a small fraction of alcohol-associated liver disease burden as <6% of patients who develop severe disease with alcohol-related hepatitis are listed for liver transplant.80,81
Ongoing Clinical Trials
Based on the data above, COVID-19 has significantly impacted alcohol use worldwide, especially in those with AUD. The short-term implications of the COVID-19 pandemic on AUD have been studied chiefly via survey analysis. Ongoing clinical trials are investigating the impact of COVID-19 on the drinking behavior of patients with underlying chronic liver disease (NCT04876443, NCT04375670). In addition, diagnostic and intervention studies investigating the outcome of behavioral intervention on AUD are also in progress (NCT05191446). These studies will help us better understand the natural history of alcohol use during a pandemic, insight into causative factors, and ways to mitigate the short-term and long-term effects of alcohol abuse in patients with and without underlying liver disease.
Key points
COVID-19 infection has caused increased mortality in patients with AUD and ALD. Though, preliminary studies have reported an increase in the total number of liver transplant listings and rates or liver transplantation in patients with ALD during COVID-19 pandemic, the increase in alcohol related transplants during the COVID-19 pandemic only reflects a small fraction of alcohol-associated liver disease burden as < 6% of patients who develop severe disease with alcohol related hepatitis are listed for liver transplant. Ongoing clinical trials are investigating the impact of COVID-19 on the drinking behavior of patients with underlying chronic liver disease in addition to outcomes of behavioral interventions on AUD.
ALD remains a significant public health issue worldwide, given an increase in the rates of advanced liver disease, including liver cirrhosis that has been observed world-wide and large disease burden with men having high rates of disability-adjusted life years lost. Advanced liver disease secondary to AUD causes a significant strain on health care systems in terms of overall cost, hospitalizations, and rates of liver transplantation in this patient population There has also been a substantial increase in morbidity and mortality rates in these patients, especially those with advanced fibrosis due to alcohol-associated liver disease. Based on results from a study on 3000 patients, only 3.8% of ALD patients are referred at early stages for intervention compared to 17% and 30% of patients with non-alcoholic fatty liver disease and viral hepatitis and earlier identification and intervention in those with early stage ALD will be required to reduce overall morbidity and mortality in this population globally.82 In addition, the medical, social, and economic impacts of ALD have risen further since the onset of COVID pandemic with higher rates of binge drinking observed in selected groups and higher rates of listing and liver transplants for ALD. Individual and population-based strategies and policies are needed to counter the detrimental use of alcohol. While pharmacological and behavioral interventions remain the cornerstone of management at the individual level, specific public health policies for effective control of excessive alcohol use and its overall impact are needed. Future analyses and studies focusing on local regulations, socioeconomic and cultural factors influencing excessive alcohol use may be required to devise better policies at the population level to reduce alcohol-related mortality and morbidity effectively and thus reduce the global burden of disease.83
Credit authorship contribution statement
Conceptualization was done by Dr. Kwo and Dr. Aslam. Literature search and original writing were done by Dr. Kwo and Dr. Aslam. Jointly the manuscript was edited and finalized.
Conflicts of interest
Paul Kwo, Advisory Board Durect, Surrozen. Stock Options Durect.
Funding
None.
References
- 1.Castro FG. Culture and Alcohol Use: Historical and Sociocultural Themes from 75 Years of Alcohol Research. [DOI] [PMC free article] [PubMed]
- 2.Park S.H., Kim D.J. Global and regional impacts of alcohol use on public health: emphasis on alcohol policies. Clin Mol Hepatol. 2020 Oct;26:652–661. doi: 10.3350/cmh.2020.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addolorato G., Abenavoli L., Dallio M., et al. Alcohol associated liver disease 2020: a clinical practice guideline by the Italian Association for the Study of the Liver (AISF) Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2020 Apr;52:374–391. doi: 10.1016/j.dld.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34:135–143. [PMC free article] [PubMed] [Google Scholar]
- 5.Samokhvalov A.V., Popova S., Room R., Ramonas M., Rehm J. Disability associated with alcohol abuse and dependence. Alcohol Clin Exp Res. 2010 Nov;34:1871–1878. doi: 10.1111/j.1530-0277.2010.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lees B., Meredith L.R., Kirkland A.E., Bryant B.E., Squeglia L.M. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020 May;192 doi: 10.1016/j.pbb.2020.172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillmore M.T., Jude R. Defining “binge” drinking as five drinks per occasion or drinking to a .08% BAC: which is more sensitive to risk? Am J Addict. 2011 Oct;20:468–475. doi: 10.1111/j.1521-0391.2011.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center of Disease Control and Prevention. Alcohol and Public Health. Available from: https://www.cdc.gov/alcohol/onlinemedia/infographics/excessive-alcohol-use.html [ Accessed Date: 11.29.2021].
- 9.WHO. International Statistical Classification of Diseases and Related Health Problems (ICD). Available from: https://www.who.int/standards/classifications/classification-of-diseases [ Accessed Date: 11.29.2021].
- 10.WHO. Global Burden of disease 2004. Available from: https://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf [ Accessed Date: 11.29.2021].
- 11.SAMHSA. 2016 National survey on drug use and health. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf [Accessed Date: 12.20.2021].
- 12.Udompap P., Kim D., Kim W.R. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015 Nov;13:2031–2041. doi: 10.1016/j.cgh.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazier T.H., Stocker A.M., Kershner N.A., Marsano L.S., McClain C.J. Treatment of alcoholic liver disease. Ther Adv Gastroenterol. 2011 Jan;4:63–81. doi: 10.1177/1756283X10378925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Shea R.S., Dasarathy S., McCullough A.J. Practice guideline committee of the American association for the study of liver diseases, practice parameters committee of the American college of gastroenterology. Alcoholic liver disease. Hepatol Baltim Md. 2010 Jan;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 15.Ramstedt M. Per capita alcohol consumption and liver cirrhosis mortality in 14 European countries. Addict Abingdon Engl. 2001 Feb;96(suppl 1):S19–S33. doi: 10.1080/09652140020021152. [DOI] [PubMed] [Google Scholar]
- 16.Mellinger J.L. Epidemiology of alcohol use and alcoholic liver disease. Clin Liver Dis. 2019 May 31;13:136–139. doi: 10.1002/cld.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellentani S., Saccoccio G., Costa G., et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997 Dec;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osna N.A., Donohue T.M., Kharbanda K.K. Alcoholic liver disease: pathogenesis and current management. Alcohol Res Curr Rev. 2017;38:147–161. [PMC free article] [PubMed] [Google Scholar]
- 19.Lieber C.S., Jones D.P., Decarli L.M. Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest. 1965 Jun;44:1009–1021. doi: 10.1172/JCI105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liangpunsakul S., Puri P., Shah V.H., et al. Effects of age, sex, body weight, and quantity of alcohol consumption on occurrence and severity of alcoholic hepatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2016 Dec;14:1831–1838.e3. doi: 10.1016/j.cgh.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basra S., Anand B.S. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol. 2011 May 27;3:108–113. doi: 10.4254/wjh.v3.i5.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palaniyappan N., Subramanian V., Ramappa V., Ryder S.D., Kaye P., Aithal G.P. The utility of scoring systems in predicting early and late mortality in alcoholic hepatitis: whose score is it anyway? Int J Hepatol. 2012;2012 doi: 10.1155/2012/624675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon K.V., Gores G.J., Shah V.H. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin Proc. 2001 Oct;76:1021–1029. doi: 10.4065/76.10.1021. [DOI] [PubMed] [Google Scholar]
- 24.Han S., Yang Z., Zhang T., Ma J., Chandler K., Liangpunsakul S. Epidemiology of alcohol-associated liver disease. Clin Liver Dis. 2021 Aug;25:483–492. doi: 10.1016/j.cld.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y.N., Fowler K.J., Hamilton G., et al. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br J Radiol. 2018 Sep;91 doi: 10.1259/bjr.20170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torruellas C., French S.W., Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014 Sep 7;20:11684–11699. doi: 10.3748/wjg.v20.i33.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dang K., Hirode G., Singal A.K., Sundaram V., Wong R.J. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020 Jan;115:96–104. doi: 10.14309/ajg.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 28.Wong T., Dang K., Ladhani S., Singal A.K., Wong R.J. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001-2016. JAMA. 2019 May 7;321:1723–1725. doi: 10.1001/jama.2019.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker R., Aithal G.P., Becker U., et al. Natural history of histologically proven alcohol-related liver disease: a systematic review. J Hepatol. 2019 Sep;71:586–593. doi: 10.1016/j.jhep.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Naveau S., Giraud V., Borotto E., Aubert A., Capron F., Chaput J.C. Excess weight risk factor for alcoholic liver disease. Hepatol Baltim Md. 1997 Jan;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 31.Jinjuvadia R., Liangpunsakul S. Translational research and evolving alcoholic hepatitis treatment consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol. 2015 Jul;49:506–511. doi: 10.1097/MCG.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adejumo A.C., Cholankeril G., Iqbal U., et al. Readmission rates and associated outcomes for alcoholic hepatitis: a nationwide cohort study. Dig Dis Sci. 2020 Apr;65:990–1002. doi: 10.1007/s10620-019-05759-4. [DOI] [PubMed] [Google Scholar]
- 33.Thompson J.A., Martinson N., Martinson M. Mortality and costs associated with alcoholic hepatitis: a claims analysis of a commercially insured population. Alcohol Fayettev N. 2018 Sep;71:57–63. doi: 10.1016/j.alcohol.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Sepanlou S.G., Safiri S., Bisignano C., et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020 Mar 1;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Younossi Z.M., Stepanova M., Younossi Y., et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69:564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 36.Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. Br Med J [Internet]. [cited 2021 Dec 24]. Available from: https://www.bmj.com/content/362/bmj.k281.
- 37.Shirazi F., Singal A.K., Wong R.J. Alcohol-associated cirrhosis and alcoholic hepatitis hospitalization trends in the United States. J Clin Gastroenterol. 2021 Feb 1;55:174–179. doi: 10.1097/MCG.0000000000001378. [DOI] [PubMed] [Google Scholar]
- 38.Rehm J., Shield K.D. Global burden of AUDs and alcohol liver disease. Biomedicines. 2019 Dec 13;7:99. doi: 10.3390/biomedicines7040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.A new divide in American death: Statistics show widening urban-rural health gap, The Washington Post [Internet]. [cited 2021 Dec 24]. Available from: https://www.washingtonpost.com/sf/national/2016/04/10/a-new-divide-in-american-death/.
- 40.Case A., Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015 Dec 8;112:15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Testino G. Are patients with AUDs at increased risk for cOVID-19 infection? Alcohol Alcohol Oxf Oxfs. 2020 Jun 25;55:344–346. doi: 10.1093/alcalc/agaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon A.M., Curtis B., Mandrekar P., Singal A.K., Verna E.C., Fix O.K. Alcohol-associated liver disease before and after COVID-19-an overview and call for ongoing investigation. Hepatol Commun. 2021 Sep;5:1616–1621. doi: 10.1002/hep4.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kranzler H.R., Soyka M. Diagnosis and pharmacotherapy of AUD: a review. JAMA. 2018 Aug 28;320:815–824. doi: 10.1001/jama.2018.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bramness J.G., Bye E.K., Moan I.S., Rossow I. Alcohol use during the COVID-19 pandemic: self-reported changes and motives for change. Eur Addiction Res. 2021;27:257–262. doi: 10.1159/000515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohal A., Khalid S., Green V., Gulati A., Roytman M. The pandemic within the pandemic: unprecedented rise in alcohol-related hepatitis during the COVID-19 pandemic. J Clin Gastroenterol. 2021 Oct 14 doi: 10.1097/MCG.0000000000001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebalancing the ‘COVID-19 effect’ on alcohol sales [Internet]. NielsenIQ. [cited 2021 Dec 24]. Available from: https://nielseniq.com/global/en/insights/analysis/2020/rebalancing-the-covid-19-effect-on-alcohol-sales/.
- 47.Grossman E.R., Benjamin-Neelon S.E., Sonnenschein S. Alcohol consumption during the COVID-19 pandemic: a cross-sectional survey of us adults. Int J Environ Res Publ Health. 2020 Dec 9;17:E9189. doi: 10.3390/ijerph17249189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myran D.T., Smith B.T., Cantor N., et al. Changes in the dollar value of per capita alcohol, essential, and non-essential retail sales in Canada during COVID-19. BMC Publ Health. 2021 Nov 25;21:2162. doi: 10.1186/s12889-021-12226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J.U., Majid A., Judge R., et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing AUD. Lancet Gastroenterol Hepatol. 2020 Oct;5:886–887. doi: 10.1016/S2468-1253(20)30251-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehm J., Kilian C., Ferreira-Borges C., et al. Alcohol use in times of the COVID 19: implications for monitoring and policy. Drug Alcohol Rev. 2020 May;39:301–304. doi: 10.1111/dar.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White A.M. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol Res Curr Rev. 2020;40 doi: 10.35946/arcr.v40.2.01. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chartier K.G., Guidry J.P.D., Lee C.A., Buckley T.D. At home and online during the early months of the COVID-19 pandemic and the relationship to alcohol consumption in a national sample of U.S. adults. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zipursky J.S., Stall N.M., Silverstein W.K., et al. Alcohol sales and alcohol-related emergencies during the COVID-19 pandemic. Ann Intern Med. 2021 Jul;174:1029–1032. doi: 10.7326/M20-7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sidor A., Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020 Jun 3;12:E1657. doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kar P., Tomfohr-Madsen L., Giesbrecht G., Bagshawe M., Lebel C. Alcohol and substance use in pregnancy during the COVID-19 pandemic. Drug Alcohol Depend. 2021 Aug 1;225 doi: 10.1016/j.drugalcdep.2021.108760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boschuetz N., Cheng S., Mei L., Loy V.M. Changes in alcohol use patterns in the United States during COVID-19 pandemic. WMJ Off Publ State Med Soc Wis. 2020 Sep;119:171–176. [PubMed] [Google Scholar]
- 57.Hanson J.D., Noonan C., Harris A., et al. Alcohol consumption during COVID among women with an existing alcohol-use disorder. Int J Environ Res Publ Health. 2021 Sep 8;18:9460. doi: 10.3390/ijerph18189460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bittermann T., Mahmud N., Abt P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. JAMA Netw Open. 2021 Jul 1;4 doi: 10.1001/jamanetworkopen.2021.18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cholankeril G., Goli K., Rana A., et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatol Baltim Md. 2021 Dec;74:3316–3329. doi: 10.1002/hep.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naughton F., Ward E., Khondoker M., et al. Health behaviour change during the UK COVID-19 lockdown: findings from the first wave of the C-19 health behaviour and well-being daily tracker study. Br J Health Psychol. 2021 May;26:624–643. doi: 10.1111/bjhp.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bollen Z., Pabst A., Creupelandt C., Fontesse S., Laniepce A., Maurage P. Longitudinal assessment of alcohol consumption throughout the first COVID-19 lockdown: contribution of age and pre-pandemic drinking patterns. Eur Addiction Res. 2022;28:48–55. doi: 10.1159/000518218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonny-Noach H., Cohen-Louck K., Levy I. Substances use between early and later stages of the COVID-19 pandemic in Israel. Isr J Health Pol Res. 2021 Aug 12;10:46. doi: 10.1186/s13584-021-00484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Killgore W.D.S., Cloonan S.A., Taylor E.C., Lucas D.A., Dailey N.S. Alcohol dependence during COVID-19 lockdowns. Psychiatr Res. 2021 Feb;296 doi: 10.1016/j.psychres.2020.113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandenberg B., Livingston M., O'Brien K. When the pubs closed: beer consumption before and after the first and second waves of COVID-19 in Australia. Addict Abingdon Engl. 2021 Jul;116:1709–1715. doi: 10.1111/add.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright C.J.C., Livingston M., Dwyer R., Callinan S. Second, third, fourth COVID-19 waves and the “pancession”: we need studies that account for the complexities of how the pandemic is affecting alcohol consumption in Australia. Drug Alcohol Rev. 2021 Feb;40:179–182. doi: 10.1111/dar.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szajnoga D., Klimek-Tulwin M., Piekut A. COVID-19 lockdown leads to changes in alcohol consumption patterns. Results from the Polish national survey. J Addict Dis. 2021 Jun;39:215–225. doi: 10.1080/10550887.2020.1848247. [DOI] [PubMed] [Google Scholar]
- 67.Sallie S.N., Ritou V., Bowden-Jones H., Voon V. Assessing international alcohol consumption patterns during isolation from the COVID-19 pandemic using an online survey: highlighting negative emotionality mechanisms. BMJ Open. 2020 Nov 26;10 doi: 10.1136/bmjopen-2020-044276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mangot-Sala L., Tran K.A., Smidt N., Liefbroer A.C. The impact of the COVID lockdown on alcohol consumption in The Netherlands. The role of living arrangements and social isolation. Drug Alcohol Depend. 2022 Feb 10;233 doi: 10.1016/j.drugalcdep.2022.109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonar E.E., Parks M.J., Gunlicks-Stoessel M., et al. Binge drinking before and after a COVID-19 campus closure among first-year college students. Addict Behav. 2021 Jul;118 doi: 10.1016/j.addbeh.2021.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Hooijdonk K.J.M., Rubio M., Simons S.S.H., et al. Student-, study- and COVID-19-related predictors of students' smoking, binge drinking and cannabis use before and during the initial COVID-19 lockdown in The Netherlands. Int J Environ Res Publ Health. 2022 Jan 12;19:812. doi: 10.3390/ijerph19020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valente J.Y., Sohi I., Garcia-Cerde R., Monteiro M.G., Sanchez Z.M. What is associated with the increased frequency of heavy episodic drinking during the COVID-19 pandemic? Data from the PAHO regional web-based survey. Drug Alcohol Depend. 2021 Apr 1;221 doi: 10.1016/j.drugalcdep.2021.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morales Chainé S., López Montoya A., Bosch Maldonado A., et al. Mental health symptoms, binge drinking, and the experience of abuse during the COVID-19 lockdown in Mexico. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.656036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weerakoon S.M., Jetelina K.K., Knell G. Longer time spent at home during COVID-19 pandemic is associated with binge drinking among US adults. Am J Drug Alcohol Abuse. 2021 Jan 2;47:98–106. doi: 10.1080/00952990.2020.1832508. [DOI] [PubMed] [Google Scholar]
- 74.Vasconcelos M., Crego A., Rodrigues R., Almeida-Antunes N., López-Caneda E. Effects of the COVID-19 mitigation measures on alcohol consumption and binge drinking in college students: a longitudinal survey. Int J Environ Res Publ Health. 2021 Sep 17;18:9822. doi: 10.3390/ijerph18189822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bor J., Basu S., Coutts A., McKee M., Stuckler D. Alcohol use during the great recession of 2008-2009. Alcohol Alcohol Oxf Oxfs. 2013 Jun;48:343–348. doi: 10.1093/alcalc/agt002. [DOI] [PubMed] [Google Scholar]
- 76.Ristic-Medic D., Petrovic S., Arsic A., Vucic V. Liver disease and COVID-19: the link with oxidative stress, antioxidants and nutrition. World J Gastroenterol. 2021 Sep 14;27:5682–5699. doi: 10.3748/wjg.v27.i34.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rutledge S.M., Schiano T.D., Florman S., Im G.Y. COVID-19 aftershocks on alcohol-associated liver disease: an early cross-sectional report from the U.S. Epicenter. Hepatol Commun. 2021 Jul;5:1151–1155. doi: 10.1002/hep4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021 Mar;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Axley P.D., Richardson C.T., Singal A.K. Epidemiology of alcohol consumption and societal burden of alcoholism and alcoholic liver disease. Clin Liver Dis. 2019 Feb;23:39–50. doi: 10.1016/j.cld.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 80.Anderson M.S., Valbuena V.S.M., Brown C.S., et al. Association of COVID-19 with new waiting list registrations and liver transplantation for alcoholic hepatitis in the United States. JAMA Netw Open. 2021 Oct 1;4 doi: 10.1001/jamanetworkopen.2021.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathurin P., Moreno C., Samuel D., et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011 Nov 10;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 82.Shah N.D., Ventura-Cots M., Abraldes J.G., et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019 Oct;17:2320–2329.e12. doi: 10.1016/j.cgh.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singal A.K., Kwo P., Kwong A., et al. Research methodologies to address clinical unmet needs and challenges in alcohol-associated liver disease. Hepatol Baltim Md. 2022 Apr;75:1026–1037. doi: 10.1002/hep.32143. [DOI] [PMC free article] [PubMed] [Google Scholar]