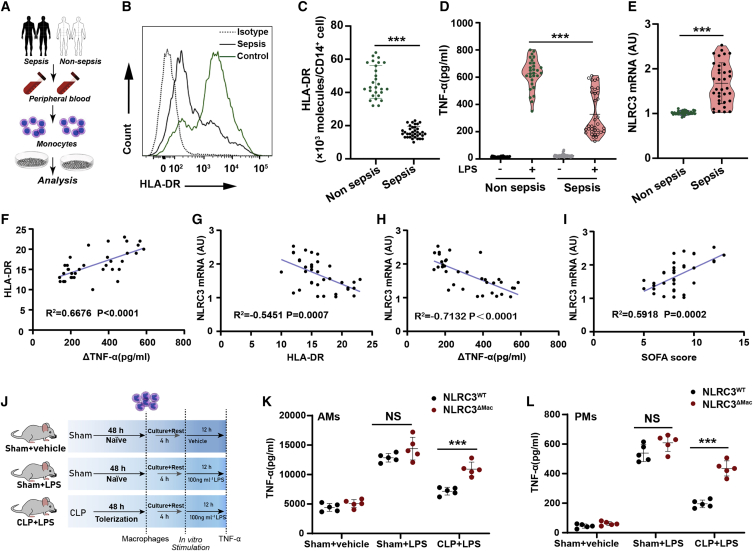
Figure 1.
NLRC3 is upregulated in clinical immunotolerant monocytes and macrophages that underwent post-CLP immunosuppression
(A) Schematic overview of experimental design for (B) to (I). (B and C) Flow cytometry assessment of HLA-DR expression on circulating monocytes from septic patients (n = 35) and non-septic donors (n = 29). (D) ELISA for TNF-α in the culture supernatants from septic patients (n = 35) and non-septic donor monocytes (n = 29) stimulated with or without LPS (10 ng/mL) in vitro for 12 h (data are presented in a violin plot and were compared with a t test). (E) NLRC3 mRNA levels in monocytes of septic patients (n = 35) and non-septic donors (n = 29). (F) Correlation assay between HLA-DR levels and change in TNF-α production (ΔTNF-α) in monocytes of septic patients (n = 35). (G–I) Correlation assay between NLRC3 levels and HLA-DR levels (G), and changes in TNF-α production (ΔTNF-α) in monocytes (H) and SOFA scores (I) of septic patients (n = 35). (J) Treatment schematic for (K) and (L). (K and L) ELISA for TNF-α in the supernatants of NLRC3WT (LysM-Cre-NLRC3fl/fl) and NLRC3ΔMac (LysM-Cre+ NLRC3fl/fl) mouse AMs (K) and PMs (L) stimulated with or without LPS in vitro (n = 5). ΔTNF-α: RPMI-stimulated samples versus LPS-stimulated samples. Circles represent individual participants. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS, not significant (two-way ANOVA or Student’s t test). See also Table S3 and Figures S1–S6.