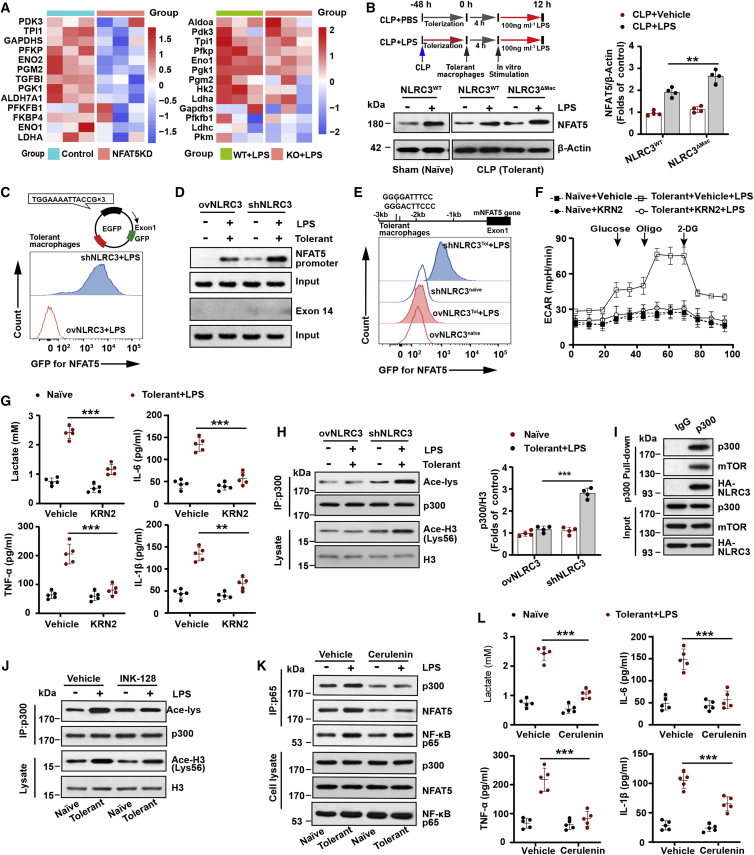
Figure 5.
p300-mediated NFAT5 signaling is required for activation of NLRC3−/−-tolerant macrophage glycolysis
(A) The heatmap closely related to glycolysis is based on RNA-seq data comparing NFAT5 shRNA (NFAT5KD) and transduced empty vector (NFAT5Ctrl) RAW 264.7 (left), and NFAT5 KO BMDMs (NFAT5 KO) and NFAT5 WT BMDMs (NFAT5WT) treated with LPS (right) from the NCBI GEO database.45 Each column in the heatmap represents the ratio of normalized expression (n = 3). (B) Immunoblot to detect NFAT5 levels in PMs with or without LPS in vitro challenge after CLP or sham operation (n = 4). (C) Tolerant RAW 264.7 with stable NLRC3 deficiency (shNLRC3) or overexpression (ovNLRC3) were transfected with NFAT5 consensus sequences fused to a GFP reporter construct. Flow cytometry was performed to analyze NFAT5-dependent GFP expression (n = 3). (D, E, and H) (D) Tolerant macrophages were stably transfected with ovNLRC3 or shNLRC3 vector and restimulated with LPS, where macrophages (naive) transfected with ovNLRC3 or shNLRC3 vector without LPS served as the control groups; ChIP assays were conducted in these cells after 90-min secondary LPS stimulation, with exon14 used as a negative control (n = 3); (E) Flow cytometry monitored the GFP expression level in these macrophages following transfection with GFP reporter system containing two NF-κB binding sites upstream of NFAT5 gene (n = 3). (H) Immunoprecipitated p300 and lysate histone H3 were analyzed with anti-acetyl-lysine and anti-acetyl-histone H3 in these cells, respectively (n = 3). (F) Seahorse XFe 24 monitored ECAR in tolerant RAW 264.7 with stable NLRC3 deficiency in the presence of KRN2 or vehicle after LPS restimulation, and in naive macrophages with stable NLRC3 deficiency in the presence of KRN2 or vehicle without LPS stimulation (n = 5). (G and L) ELISA for lactate production and TNF-α, IL-6, and IL-1β in tolerant BMDMs of NLRC3ΔMac (LysM-Cre+ NLRC3fl/fl) mice in the presence of KRN2 (G) or cerulenin (L) or vehicle after undergoing LPS restimulation, and in naive macrophages of NLRC3ΔMac in the presence of KRN2 (G) or cerulenin (L) or vehicle in the absence of LPS stimulation (n = 5). (I) Human embryonic kidney (HEK) 293T cells were transfected with the p300, HA-NLRC3, and mTOR plasmids. p300 immunoprecipitates were analyzed for p300, HA, and mTOR expression by immunoblot (n = 3). (J) Tolerant macrophages with stable NLRC3 deficiency treated with vehicle or INK-128 before LPS rechallenge (n = 3). Naive macrophages with stable NLRC3 deficiency without LPS treatment served as the control group. Immunoprecipitated p300 and lysate histone H3 were analyzed with anti-acetyl-lysine and anti-acetyl-histone H3, respectively. (K) Tolerant macrophages with stable NLRC3 deficiency were treated with vehicle or cerulenin before LPS rechallenge, with naive macrophages transfected with shNLRC3 without undergoing LPS treatment as the control group. Cell lysates were immunoprecipitated (IP) using anti-p65 antibody. The immunoprecipitates and cell lysates were immunoblotted for p300, NFAT5, and p65 (n = 3). Circles represent individual mice. Graphs show the mean ± SE of experiment replicates and are representative of at least three independent experiments. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS, not significant (two-way ANOVA or Student’s t test). See also Figures S16–S18.