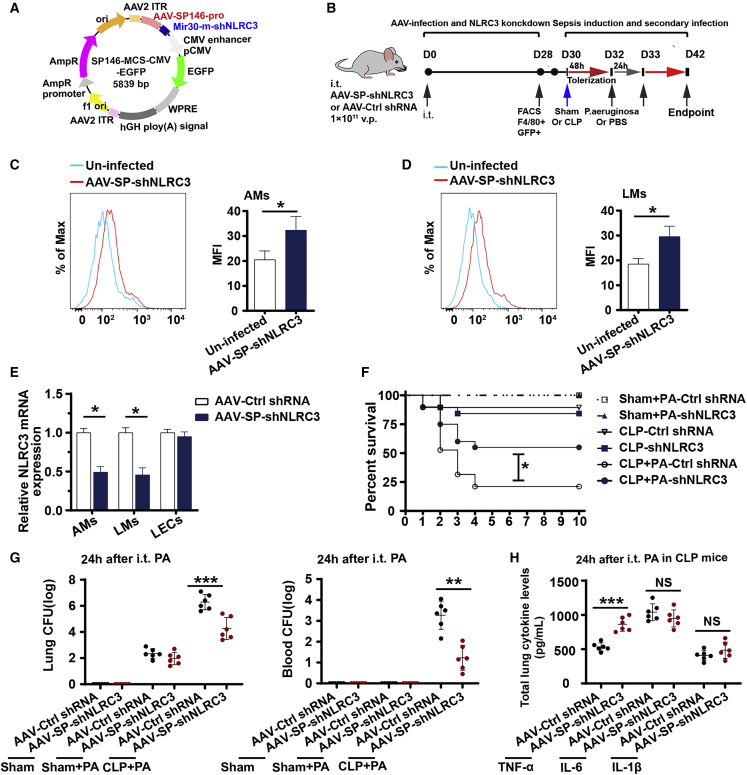
Figure 6.
NLRC3 gene therapy targeting intrapulmonary macrophages improves sepsis-induced suppression of lung immune defense
(A) Full sequence map for AAV-SP146-miR30-shNLRC3-eGFP (AAV-SP-shNLRC3). (B) Schematic overview of experimental design for (C) to (H). Mice were administrated intratracheally with AAV-SP-shNLRC3 or AAV-Ctrl shRNA (nonsense control shRNA), and 28 days later were subjected to CLP or sham operation and secondary intrapulmonary P. aeruginosa infection. (C and D) Flow cytometric analysis of GFP expression in AMs from bronchoalveolar lavage fluid (C) and in lung macrophages (LMs) from homogenates (D) of uninfected and AAV-SP-shNLRC3-infected mice on day 28. (E) RT-PCR detected NLRC3 expression in AMs, LMs, and lung epithelial cells of AAV-SP-shNLRC3 or AAV-Ctrl shRNA-infected mice. (F–H) Surviving mice with AAV-SP-shNLRC3 or AAV-Ctrl shRNA were subjected to secondary intrapulmonary P. aeruginosa infection after CLP or sham operation: Kaplan-Meier survival curves (n = 19 mice/group) (F), bacterial loads in the lung (left) and blood (right) (G), ELISA for TNF-α, IL-1β, and IL-6 in lung homogenate (H); survival curves were monitored for 10 days, and samples were collected after the secondary infection challenge. i.t., intratracheal. Circles represent individual mice. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS, not significant (two-way ANOVA or Student’s t test and log-rank test for survival).