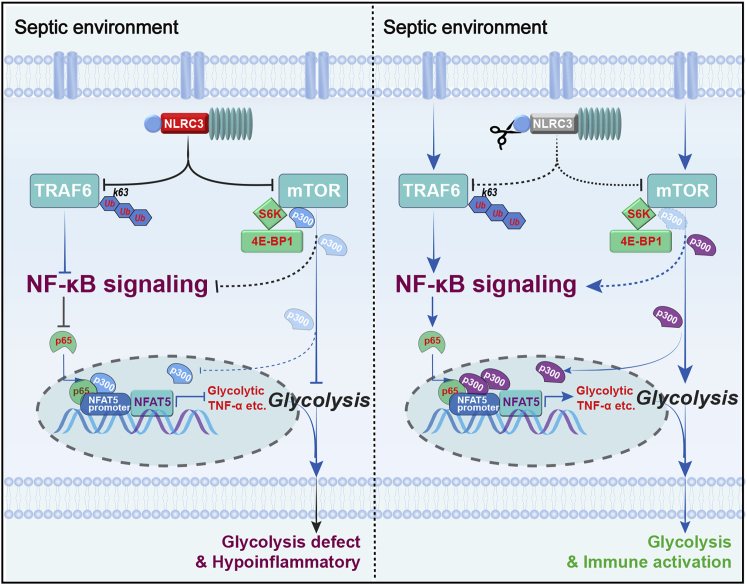
Figure 7.
Schematic model depicting how NLRC3 drives glycolytic defects and septic immunosuppression
(Left) NLRC3 expression of immunosuppressive macrophages increases in the context of sepsis-induced immunosuppression. On one hand, increased NLRC3 inhibits NF-κB translocation co-induced by both TRAF6- and mTOR-dependent signaling. On the other hand, NLRC3 association with mTOR and p300 inhibits mTOR-mediated phosphorylation dependent on p300 activity. Finally, the p300 activity and the NF-κB binding to the NFAT5 promoter region was decreased, thereby preventing the NF-κB-NFAT5 complex from controlling the expression of genes encoding glycolytic enzymes and proinflammatory cytokines. (Right) Macrophage-specific NLRC3 deletion increases the p300 activity and further enhances NF-κB binding to the NFAT5 promoter region following NF-κB translocation, and thereby the enhanced NF-κB-NFAT5 complex elicits fine-tuning of proinflammatory cytokine production and glycolysis while the septic host achieves an enhanced protective immune response against secondary bacterial challenge.