Figure 2: Functional outcomes and patient- and physician-reported measures.
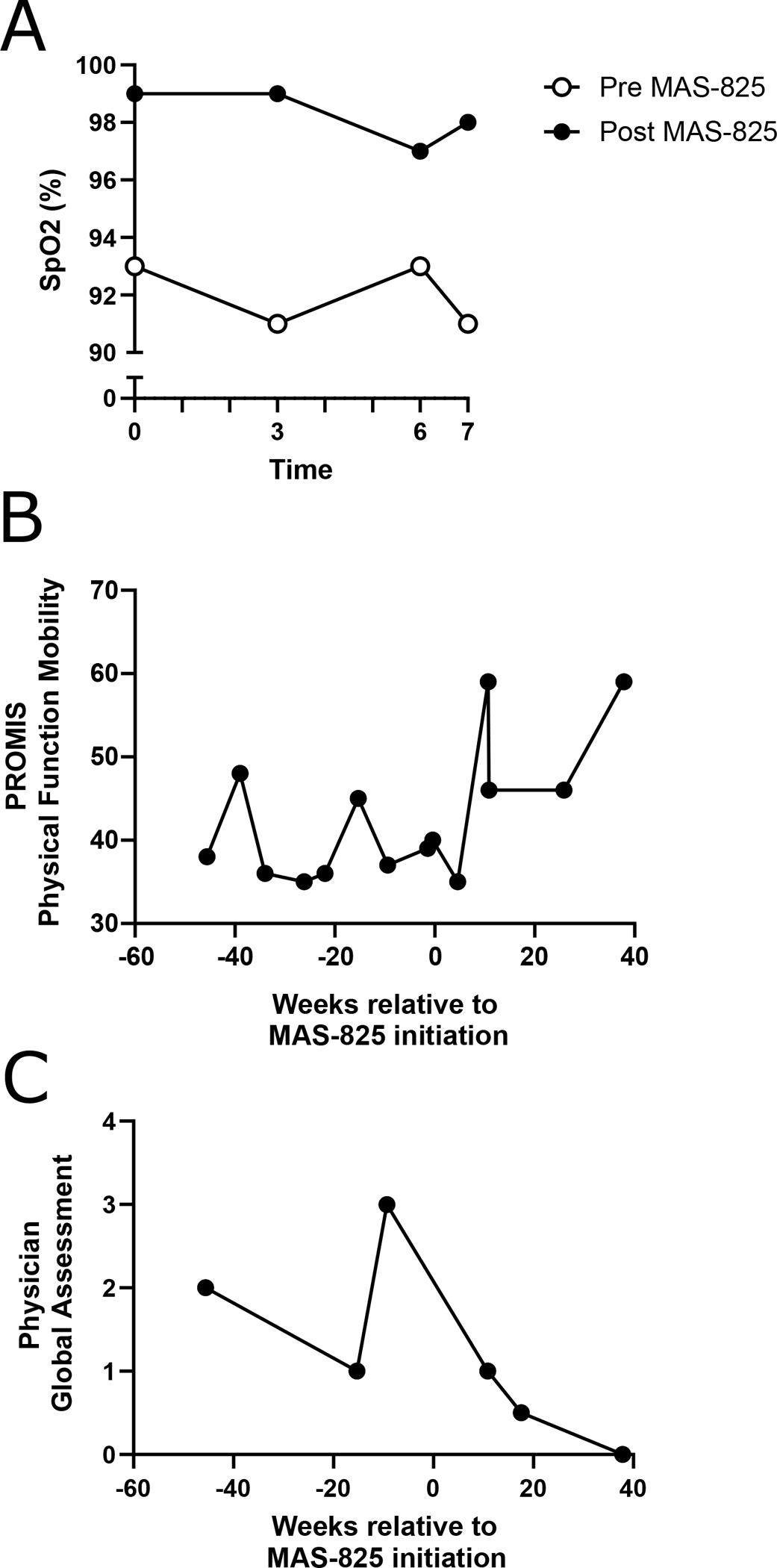
(A) Oxygen saturation (SpO2) during 6-minute walk test and 1 minute post-test, performed 3 days prior to and 7.5 weeks after initiation of MAS-825. (B) Patient-reported scores on the PROMIS Pediatric Short Form V1.0 Physical Function Mobility Questionnaire for all available timepoints 45 weeks prior to and 45 weeks after initiation of MAS-825, with higher scores indicating better mobility. (C) Physician global assessment as reported by the treating rheumatologist for all available timepoints 45 weeks prior to and 45 weeks after initiation of MAS-825, with lower scores indicating lower disease activity.
