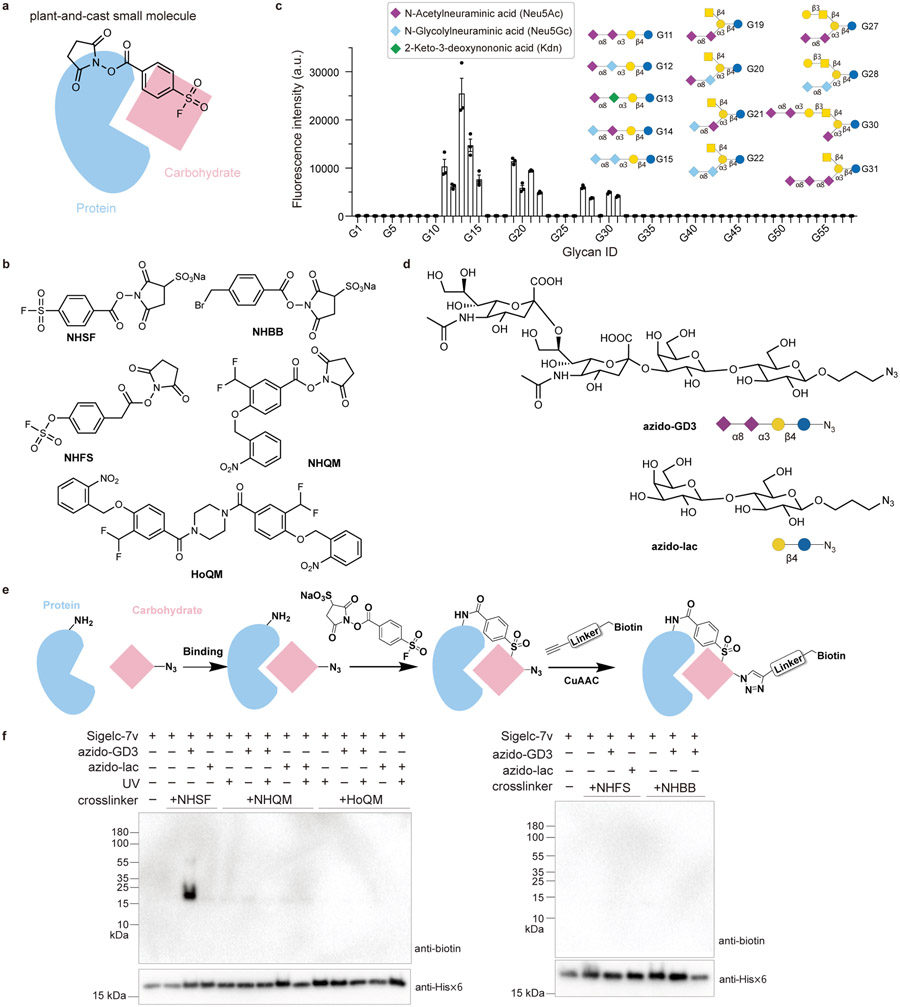
Fig. 1. Sulfonyl fluoride was identified as a suitable functional group for cross-linking carbohydrate through proximity-enabled reactivity using a strategy involving plant-and-cast small molecule cross-linkers.
a) Scheme of the strategy: when the plant-and-cast small molecule cross-linker is added to protein-carbohydrate complex, the succinimide ester of the cross-linker reacts rapidly with Lys sidechains of the protein, placing the less reactive test functionality in close proximity to carbohydrate. If the functionality reacts with carbohydrate driven by proximity-enabled reactivity, the carbohydrate will be covalently cross-linked to the protein for detection. b) Chemical structures of five cross-linkers tested to cross-link protein with carbohydrate. c) Binding analysis of the refolded Siglec-7v with the glycosphingolipid glycan microarray, confirming Siglec-7v binding with the Neu5Acα2–8Neu5Ac-terminating glycan ligands. n=3 independent samples. The bar height and error bar represent mean ± SEM. d) Chemical structures of azido-GD3 for binding with Siglec-7v and the negative control azido-lac. e) Scheme showing the cross-linking and detection procedures. Siglec-7v was incubated with azido-GD3 for binding, after which the cross-linker was added to cross-link. Biotin was subsequently appended onto azido-GD3 via click chemistry for detection of the cross-linked GD3. f) Among the five tested cross-linkers, only NHSF cross-linked Siglec-7v with azido-GD3.