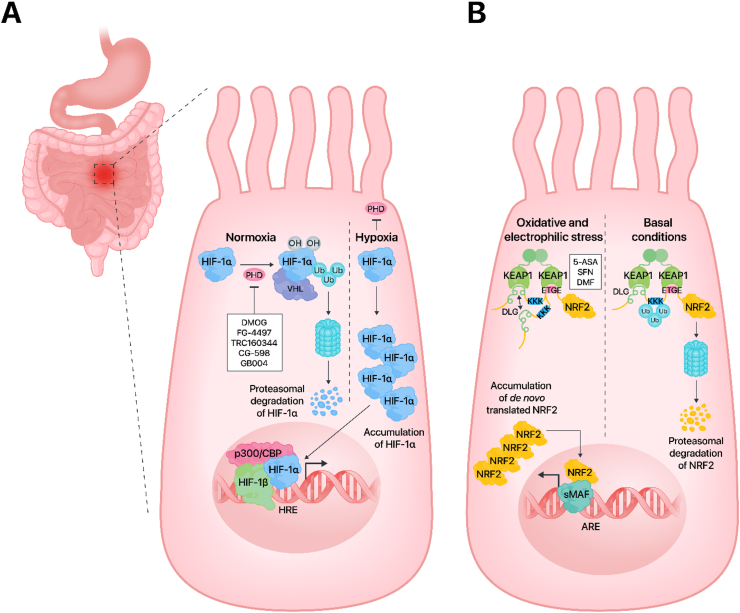
Fig. 2.
Modulation of HIF-1 and NRF2 signaling pathways. (A) Prolyl hydroxylases (PHD) are oxygen concentration-dependent enzymes, which under normoxic conditions hydroxylate the α-subunit of HIF-1 to initiate its immediate proteasomal degradation. Hypoxia and PHD inhibitors block the activity of PHDs (by reducing the hydroxylation step), which leads to HIF-1α stabilization and nuclear translocation. The complex comprising a HIF-1α/β heterodimer and p300/CBP drives the expression of hypoxia-inducible target genes. Albeit intestinal epithelial cells are shown, it is important to note that HIF is not solely restricted to these cells but is also active in immune cells like macrophages, dendritic cells, and T- and B-lymphocytes (B) Under non-stressed conditions, the binding of NRF2 to KEAP1 exposes NRF2 lysines for CUL3-dependent ubiquitination, which targets NRF2 to proteasomal degradation. In the presence of oxidative stress and/or electrophiles, such as 5-ASA, SFN and DMF, conformational changes prevent NRF2 ubiquitination. De novo synthesized NRF2 translocates into the nuclei and triggers transcription of its target genes. Abbreviations: 5-ASA, 5-aminosalicylic acid; ARE, antioxidant response element; DLG, a low affinity binding motif for KEAP1; DMF, dimethyl fumarate; DMOG, dimethyloxallyl glycine; ETGE, a high affinity binding motif for KEAP1; HIF-1, hypoxia-inducible factor 1; HRE, hypoxia response element; KEAP1, Kelch-like ECH-associated protein 1; NRF2, nuclear factor erythroid 2-related factor 2; p300/CBP, acetyltransferases p300 and CREB binding protein; PHD, prolyl hydroxylase; SFN, sulforaphane; sMAF, small musculoaponeurotic fibrosarcoma protein; Ub, ubiquitin; VHL, von Hippel-Lindau tumor suppressor protein.