Figure EV5. Regulation of MASTL activity by mTORC1.
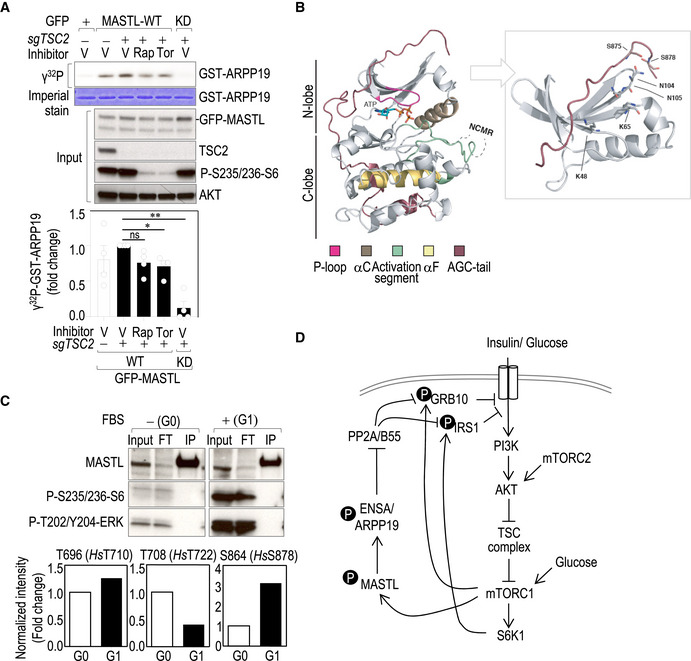
- In vitro kinase assay using GFP‐hMASTL immunocomplexes from asynchronous TSC2 knockout cells (sgTSC2) or control cells treated with the indicated inhibitors for 1 h. Immunoprecipitation of GFP alone was used as a control. GST‐ARPP19 was used as a substrate. The upper panel shows the radiograph of ARPP19 phosphorylation in the different conditions accompanied by the immunoblots of the whole‐cell extracts. Quantification of the relative kinase activity of MASTL is shown in the lower graph, in which MASTL activity in sgTSC2 cells was set as 1. Data are mean ± SEM from four independent experiments. Significance determined by paired Student's t‐test (ns, not significant; *P < 0.05; **P < 0.01).
- A homology structural model showing the position of S875 and S878 in the regulatory C‐tail domain of human MASTL. Ribbon diagram of the MASTL kinase domain (without the residues 181–727 coding for the NCMR; left panel) in complex with ATP. The protein fold displays the standard bilobal structure (N‐lobe and C‐lobe) with its respective AGC C‐tail (shown in brown). Please see the associated key for common structural kinase domain components. Molecular details for the phospho‐binding pocket of MASTL (right panel). Using the homology‐modeled AGC C‐tail of MASTL, it is possible to determine that amino acids Lys48, Lys65, Asn104, and Asn105 (stick representation) are positioned such that they could interact with the phosphorylated tail/liker reside (pSer875) and the mTOR‐phosphorylation site (pSer878). Note, in both panels, the MASTL protein has been homology‐modeled using MODELLER based on the structures of MASTL (PDB 5LOH2; https://www.rcsb.org/) for the kinase domain and of PKA and RSK2 (PDBs 1ATP and 4EL9, respectively) for the AGC C‐tail.
- Mouse embryonic fibroblasts (MEFs) were synchronized in G0 by serum starvation and then released for 6 h in the presence of serum (G1). Endogenous Mastl was immunoprecipitated with a specific antibody against Mastl (Abgent) and subjected to mass spectrometry to identify phosphorylated residues in Mastl in the indicated conditions. The upper panel shows the immunoblot of the immunoprecipitates and inputs probed with the indicated antibodies. The lower graphs show the relative quantification of the indicated phosphopeptides in both conditions.
- A model for the role of the MASTL‐ENSA/ARPP19‐PP2A/B55 pathway in the negative feedback loop that controls AKT activity in response to mTORC1‐S6K1 signaling. For simplicity, only the phosphoresidues in the negative feedback loop are shown.
