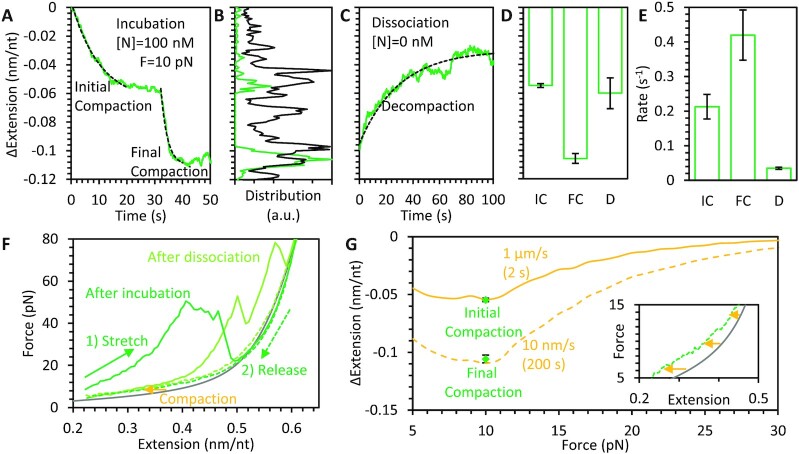
Figure 2.
Compaction of ssDNA by FL N protein. (A) ssDNA held at 10 pN constant tension is incubated and immediately bound and compacted by 100 nM N protein. ssDNA extension does not decrease monotonically, but temporarily pauses before continuing to compact after a variable time delay. Both compaction steps are well fit by exponential equations (dashed lines). (B) Instantaneous values of ssDNA compaction during protein incubation for both single experiment (panel A data, green) and all experiments summed (N = 10, black) show peaks at partial compaction, indicating consistent pausing before reaching full compaction. (C) After 100 s N protein incubation, free protein is removed from the sample, allowing for protein dissociation without replacement, resulting in partial decompaction (green, same experiment as A) well fit by an exponential dependence (dashed line). Amplitudes (D) and rates (E) of exponential fits to initial (IC) and final compaction (FC) during incubation (panel A) and decompaction (D) during dissociation (panel C) are averaged for all experiments (N = 10). Compaction amplitudes of 0.05 and 0.1 nm/nt are consistently observed after the first compaction step and last compaction step, respectively, in agreement with the two largest peaks in the instantaneous compaction histogram (panel B). The final compaction step is twice as fast as the initial step on average. Decompaction consistently occurs over a 50 s timescale but only results in partial decompaction. (F) The ssDNA–protein complex is stretched immediately after protein incubation (solid green), exhibiting a large degree of substrate compaction (reduced extension). At high force, these protein-mediated compacted structures are destabilized, resulting in ssDNA extending to its protein-free length (gray line, FJC fit from Figure 1D). Decreasing the substrate extension to its original value (dashed green) displays reduced, but non-zero, compaction. Stretching the ssDNA after 200 s dissociation (light green) instead of directly after incubation (dark green) exhibits reduced but non-zero compaction, consistent with partial dissociation. (G) The decrease in extension for ssDNA–protein complexes after stretching (inset shows magnified view of panel F) is calculated as a function of force and averaged over N = 5 experiments. The decrease in extension is minimal at high force but increases as force is reduced. When the substrate extension is quickly reduced (1 μm/s, 2 s timescale), the decrease in extension at 10 pN is equal to the initial compaction step during incubation (panel A). When the ssDNA substrate extension is slowly reduced (10 nm/s, 200 s timescale), the decrease in extension is larger and comparable to final compaction.