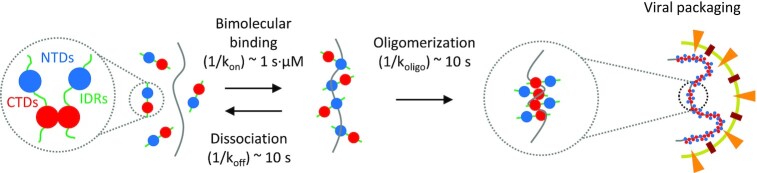
Figure 8.
Model of multistep process of N protein binding and packaging vRNA. N protein is abundant in the host cell, existing primarily as a dimer, mediated by contacts between the CTDs with the NTDs separated by the linker IDRs. When N protein encounters viral RNA, bimolecular binding occurs, primarily mediated by the NTDs. At micromolar concentrations, the RNA substrate is saturated on a second time scale (provided enough protein is present to occupy all available binding sites), with each individual bound protein remaining on the RNA substrate for tens of seconds. While the RNA is saturated with N protein, the local concentration of protein is extremely high, allowing for higher order oligomeric states to form. The structure of the RNA-protein complex is determined by interactions involving CTDs of neighboring proteins. Once in this oligomeric state, binding of the protein oligomers is very stable, preventing individual proteins from dissociating from the substrate and the RNA from decompacting. The nearly 30 kb viral RNA genome (∼16 μm extended length) is compacted over 100× in order to fit inside the ∼80 nm diameter viral particle.