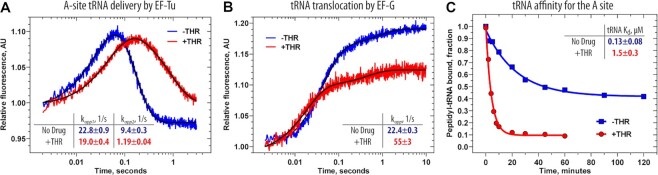
Figure 5.
Ribosome-bound THR impairs the elongation cycle. (A) Pre-steady state kinetics of A-site tRNA binding upon interaction of the ternary complex EF-Tu•GTP•Phe-tRNAPhe(Prf16/17) (0.1 μM) with the 70S initiated ribosomes (0.4 μM) containing fMet-tRNAifMet in the P site. Shown are time courses in the absence (blue) and presence (red) of THR. (B) Pre-steady state kinetics of translocation upon interaction of the 70S pre-translocation ribosome complexes (60 nM) containing deacylated-tRNAifMet in the P site and fMet-Phe-tRNAPhe(Prf16/17) in the A site with EF-G (2 μM). Color scheme is the same as in panel A. Each time course represents the average of five to seven experimental replicates. Standard deviations associated with the kinetics were calculated using GraphPad Prism software. (C) Time courses of dissociation of peptidyl-tRNA from the A site of the 70S pre-translocation ribosome complexes in the absence (blue) or presence (red) of THR.