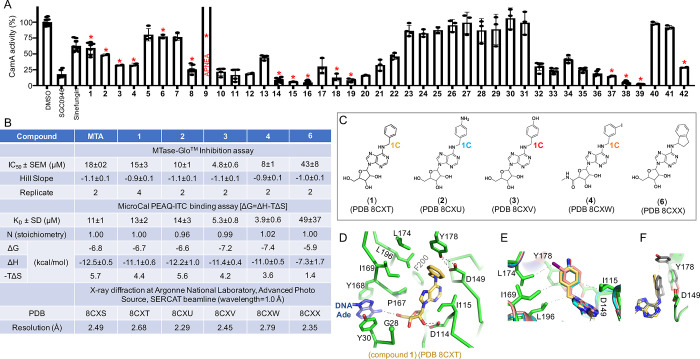
Figure 3.
Structures of N6-benzyladenosine (1) and its related one-carbon analogs. (A) Relative inhibition of CamA activity at a single inhibitor concentration of 10 μM, in the presence of 40 μM co-substrate SAM. Compounds indicated by a red asterisk were chosen for further study. (B) Summary of inhibition (IC50 and Hill slope), dissociation constant (KD) measured by isothermal titration calorimetry, and X-ray information (PDB accession numbers and corresponding resolutions). (C) Additions at the N6 position of adenosine: benzyl (1), 4-aminobenzyl (2), 4-hydroxybenzyl (3), 3-iodobenzyl (4), and (1R)-indanyl ring (6). (D) N6-benzyl moiety (1) is surrounded by hydrophobic residues (PDB 8CXT). (E) Superimposition of benzyl-(PDB 8CXT), 4-aminobenzyl-(PDB 8CXU), 4-hydroxybenzyl-(PDB 8CXV), and 3-iodobenzyl-adenosines bound with CamA (PDB 8CXW). (F) Superimposition of benzyl- (PDB 8CXT) and indanyl-adenosines (PDB 8CXX). Tyr178 adopts two alternative conformations with the indane moiety.