Figure 5.
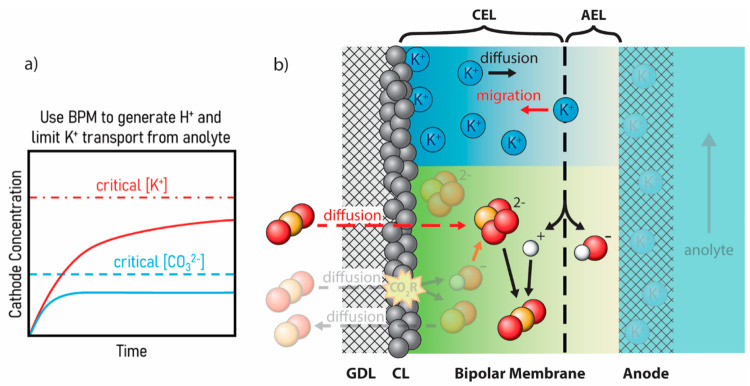
(a) Plot of cathode concentration versus time showing the general trends of K+ and CO32– concentrations at the cathode when a BPM is used. (b) Schematic showing effects of a BPM on K+-comigration past the membrane by limiting free ion transport and electro-osmotic drag. Additionally, CO32– concentrations are reduced by combining with H+ formed at the BPM junction to regenerate CO2. While changing the MEA recipe delays the accumulation of ions, it does not necessarily prevent critical concentrations from being reached.