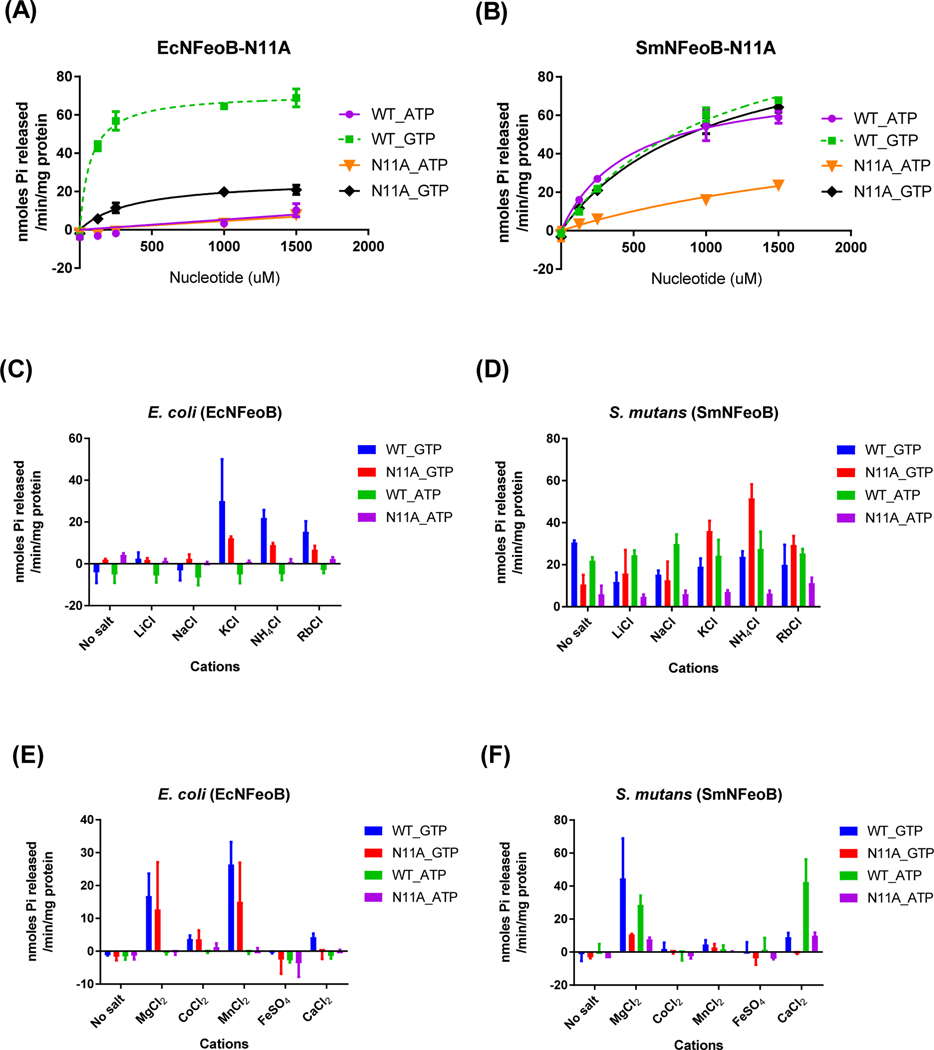
Figure 4.
Mutation of Asn11 alters nucleotide-specificity of bacterial FeoBs. The reaction rates for E. coli-N11A mutant (A) and S. mutans-N11A mutant (B) with ATP (● and ▼) and GTP (■ and ◆) as substrates were measured as described in Materials and Methods. Cation dependence profiles were not changed by the mutation in EcNFeoB (C), whereas the N11A mutant of SmNFeoB became more dependent on potassium (D). There was no difference in the profile of divalent cation dependence between the wild type and mutant in both EcNFeoB and SmNFeoB (E and F). (C and D) The enzyme reaction buffer alone (no salt) contained 5 mM MgCl2 and 10 mM NaCl. (E and F) The enzyme reaction buffer contained either 10 mM NaCl and 200 mM KCl (E) or only 10 mM NaCl (F). The error bars represent the standard deviation.