Figure 1.
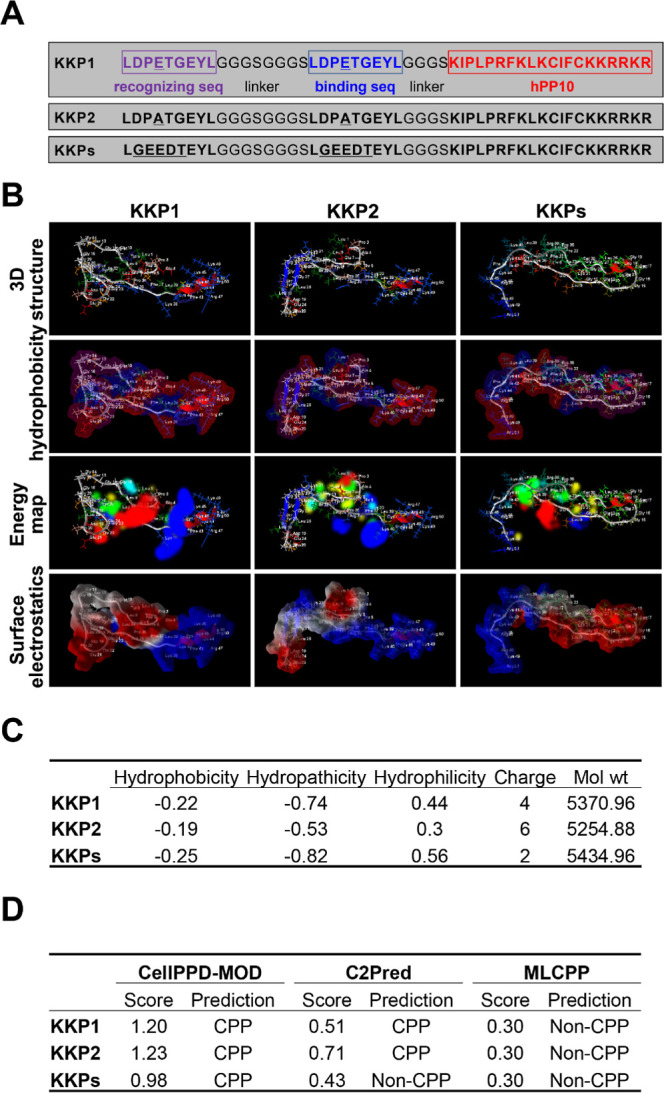
Peptide KKP secondary structure prediction. (A) Sequence of the designed PROTAC degrader KKP, residues in purple and the red text correspond to the recognition site and the binding site, respectively, and residues highlighted in red correspond to CPP-hPP10. (B) RoseTTAFold-predicted three-dimensional structure, hydrophobicity, energy map, and surface electrostatics of the peptide KKP illustrated using a Molegro Molecular Viewer. (C) Key physicochemical properties including the hydrophobicity, hydropathicity, and hydrophilicity of the peptide KKP predicted using a CellPPD-MOD online server. (D) Cell penetration property prediction of the peptide KKP conducted using CellPPD-MOD, C2Pred, and MLCPP.
