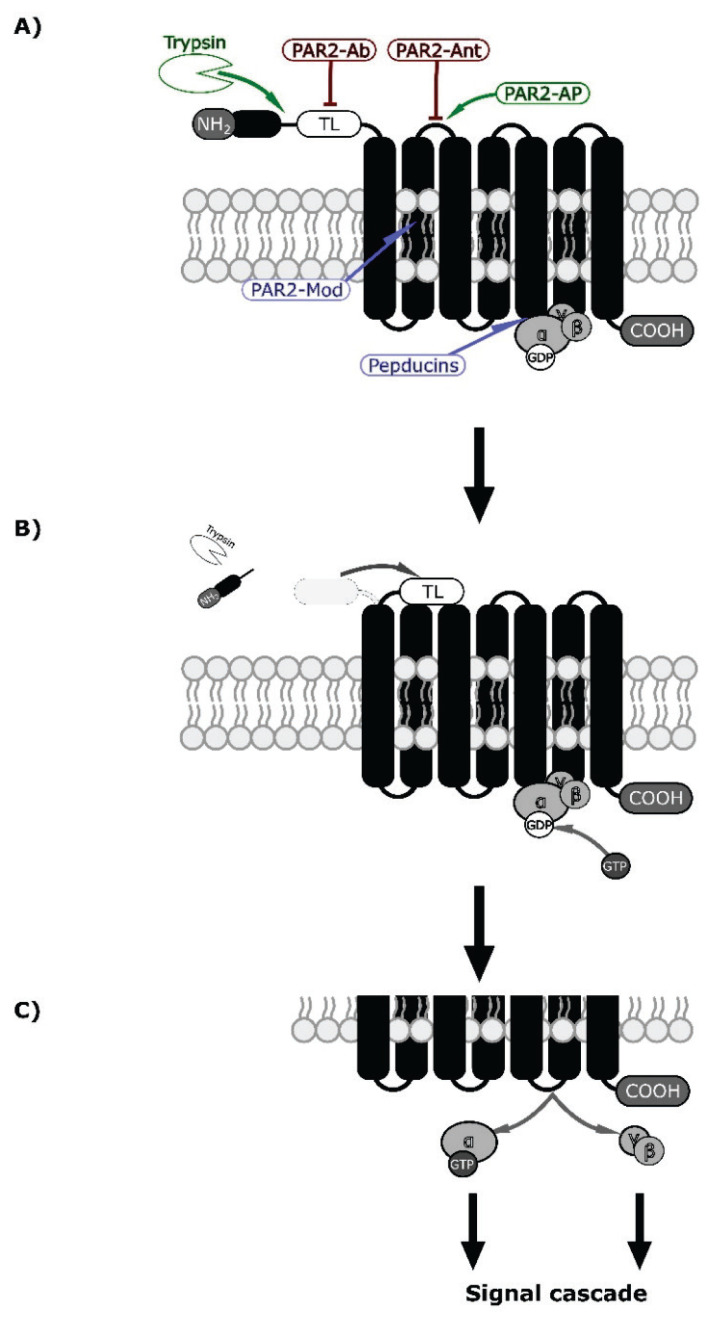
Fig. 2.
PAR2 mechanism of activation by specific proteolysis [9] and putative binding sites of drugs (agonists are green, antagonists red and modulators blue) [89,90]. (A) PAR2 receptor consists of N-terminal end (NH2 and peptide) followed by tethered ligand (TL), 7 transmembrane domains (black columns bound together by 6 loops), and C-terminal (COOH). This receptor is coupled to the inactive G-protein (a group composed of α, β, and γ domains, with attached GDP to the α domain) in a nucleotide-binding pocket on the third intracellular loop [9,97]. The enzyme (white indented ellipsis) cuts between the N-terminal and tethered ligands. At the same level, PAR2-AP binds to the first extracellular loop and causes non-enzymatic activation (TL works similarly on a different PAR [9]. (B) N-terminal end is cleaved off, and the tethered ligand is uncovered. This results in a conformational change and receptor activation by the tethered ligand. In both mechanisms, the activation of the receptor is then transferred to the G-protein, resulting in the release of GDP and replacement by GTP [98]. (C) The α-subunit with the GTP is separated from βγ -subunits, and both can induce intracellular signal cascades [97,98].