Abstract
Objective
The aim of this study was to evaluate the effectiveness of metagenomic next-generation sequencing (mNGS) for the diagnosis of Pneumocystis jirovecii Pneumonia (PCP) in critically pediatric patients.
Methods
Seventeen critically pediatric patients with PCP and sixty patients diagnosed with non-PCP pneumonia who were admitted in pediatric intensive care unit between June 2018 and July 2021 were enrolled. Conventional methods and mNGS for detecting Pneumocystis jirovecii (P. jirovecii) were compared. The patients’ demographics, comorbidities, laboratory test results, antibiotic treatment response and 30 day mortality were analyzed.
Result
The mNGS showed a satisfying diagnostic performance with a sensitivity of 100% in detecting P. jirovecii compared with Gomori methenamine silver staining (5.9%), serum (1,3)-β-D-glucan (86.7%) and and LDH (55.6%). The diagnostic specificity of mNGS for PCP was higher than that of serum BDG (56.7%) and LDH (71.4%). In PCP group, over one thirds’ cases had mixed infections. Compared with survivors, non-survivors had higher stringently mapped read numbers (SMRNs) in bronchoalveolar lavage fluid (BALF) sample (P < 0.05), suggesting SMRNs were closely associated with the severity of response. The detection for P. jirovecii by mNGS both in BALF and blood samples reached a concordance rate of 100%, and the SMRNs in the BALF were remarkably higher than that in blood samples. Initial antimicrobial treatment was modified in 88.2% of PCP patients based on the mNGS results.
Conclusion
The mNGS is a potential and efficient technology in diagnosing PCP and shows a satisfying performance in the detection of co-pathogens. Both blood and BALF samples for mNGS are suggested for the presumptive diagnosis of PCP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12941-023-00555-5.
Keywords: Metagenomics next generation sequencing, Pneumocystis jirovecii pneumonia, Critically pediatric patients, Diagnosis
Introduction
Pneumocystis jirovecii (P. jirovecii) is a common opportunistic infection which causes Pneumocystis jirovecii pneumonia (PCP) in immunocompromised population [1]. PCP is the important cause of death in hospitalized adults (13%) and children (29%) among HIV-infected people [2]. In recent years, there is an increasing incidence of PCP related to non-HIV patients, such as underlying malignancy, post-organ transplantation, kwashiorkor, treatment-related immunosuppression and/or concomitant use of corticosteroids [3, 4]. In children, the mortality of PCP with leukemia is 28–53%, and 17–30% in children with AIDS [5, 6]. Without chemoprophylaxis, up to 25% of pediatric oncology patients receiving chemotherapy will develop PCP [7]. PCP is one of the most common infectious diseases that cause children to die [8].
The diagnosis of PCP requires a combination of clinical manifestations, radiological findings and microbiological tests [9–12]. All signs and symptoms of PCP are non-specific. When the immunosuppressive host clinically presents chills, dry cough, shortness of breath, weight loss and progressive dyspnea, the possibility of PCP should be given priority [13]. Notably, compared to children older than 6 months, clinical progression of PCP in the children aged one to six months is slower [4]. The non-specific feature increases the complexity of PCP diagnosis in critically pediatric patients. Therefore, accurate and rapid diagnosis is essential for the prognosis of PCP patients.
Currently, the laboratory identifications of P. jirovecii contain classical morphology examinations and molecular methods [14]. P. jirovecii still cannot be reliably grown in vitro [15]. According to the characteristic cysts and trophozoites found under the specific staining of bronchoalveolar lavage fluid (BALF), induced sputum and other specimens, the microscopic examination of respiratory tract specimens can be used as the gold standard for the diagnosis of PCP. However, the sensitivity of routine staining is low, and its negative report is not enough to exclude the diagnosis of PCP [16]. Moreover, immunofluorescence staining is not routinely performed in many hospitals. Recently, the polymerase chain reaction (PCR) method has been considered a promising technology for detecting P. jirovecii, which has an excellent sensitivity (94–99%) and specificity (89–93%), even in specimens with low pathogen load [17]. However, due to the limitation of the genus-specific targeting regions primers, PCR methods still have difficulty in identifying mixed infections [18, 19].
Metagenomics next-generation sequencing (mNGS) is an unbiased pathogen detection and molecular technology of nucleic acid sequencing with high-throughput in a single assay, which has been considered as a promising microbial identification technology in infectious diseases [20]. Recently, its diagnostic ability on detecting a wide range of pathogens has been highlighted in several studies [21, 22]. We once reported a case about rapid and precise diagnosis of pneumonia coinfected by P. jirovecii and Aspergillus fumigatus assisted by next-generation sequencing in a patient with systemic lupus erythematosus [23]. However, the diagnostic performance of mNGS for PCP by BALF and blood specimens in non-HIV critically pediatric patients has rarely been reported.
In our study, we described the performance of mNGS of BALF and blood samples for detecting P. jirovecii in non-HIV critically pediatric patients.
Methods
Study participants
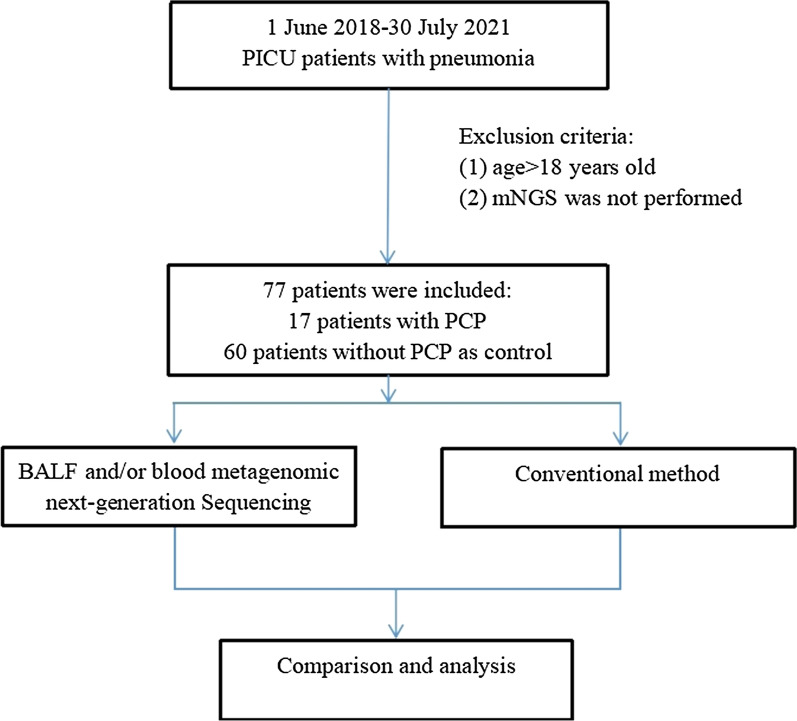
In this retrospective study, we consecutively enrolled 77 pneumonia patients who were admitted to the pediatric intensive care unit (PICU) of The First Affiliated Hospital of Sun Yat-sen University, from June 1st, 2018 to July 30th, 2021. According to the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) consensus definitions of invasive fungal diseases (IFDs) [24], the patients were divided into an observation group (PCP proven and probable diagnosed group) and a control group (non-PCP group). The details were as follows: (1) non-HIV immunosuppressed hosts; (2) accompanied by fever or dry cough, shortness of breath; (3) Chest computed tomography (CT) showed multiple ground-glass interstitial exudation, reticulate or consolidated shadows in both lungs; (4) serum (1,3)-β-D-glucan (Serum BDG) positive (> 60 pg/ml) twice; and (5) P. jirovecii trophozoites (and/or cysts) were microscopically identified following Gomori methenamine silver staining. Clinical diagnosis was made if the aforementioned items (1)–(4) were met, and confirmed diagnosis was made if items (1)–(5) were met. The clinical comprehensive diagnosis of PCP or non PCP was made by two senior expert pulmonary doctors (YJL and WT) after discussion with the medical team based on clinical symptoms, laboratory findings, chest radiology, microbiological tests and treatment responses. Patients were excluded if they met any of the following criteria: (1) HIV infection; (2) mNGS was not performed; (3) age > 18 years old; (4) medical record was incomplete (Fig. 1). The study was approved by The First Affiliated Hospital of Sun Yat-sen University and was in line with the Declaration of Helsinki.
Fig. 1.
Flowchart of case selection. A total of 77 pneumonia cases in PICU were selected for further analysis. PICU pediatric intensive care unit
Sample collection and etiological diagnosis
BALF was collected according to the guidelines [25]. After elimination of contraindications, all patients underwent bronchoscopy under intravenous combined anesthesia or 2% lidocaine topical anesthesia. After the same amount of normal saline was injected into the affected bronchial segment in several times, BALF was aspirated under negative pressure for relevant tests.
At the same time, BALF mNGS and conventional methods were used to detect pathogens in all patients. BALF and peripheral blood specimens were simultaneously submitted for etiological examination. In this study, microbiologic tests for P. jirovecii included serum (1,3)-β-D-glucan (Serum BDG), lactate dehydrogenase (LDH), Gomori methenamine silver (GMS) staining and mNGS. Other etiological laboratory diagnosis also included traditional culture methods and antigen or antibody detections.
Sample processing and DNA extraction for mNGS
Volumes of 600 µL of each patient’s BALF was taken and mixed with lysozyme and glass beads. Then, the mixture was placed inside a vortex mixer's horizontal platform and stirred intensely for 30 min at 2800–3200 rpm. For nucleic acid extraction from BALF, we transferred 200 µL of supernatant into a 2 mL centrifuge tube. Volumes of 3–5 mL of patients’ blood were centrifuged at 3500 rpm for 10 min at 4 °C for plasma separation. For DNA extraction from blood, we transferred 200 µL of plasma into a 2 mL sterile tube. Then the IDseq TM Micro DNA kit (Vision medicals, VM002-50D, China) was used to extract DNA based on standard procedures [26].
Library preparation and sequencing construction
DNA libraries were builded via transposase-based methodology. After purification and size selection, the concentration of the library was measured by using a Qubit instrument before pooling. Pooled libraries were sequenced on an Illumina NextSeq 550 system using a 75 bp, single-end sequencing kit. The qualified results had no fewer than 10 million reads obtained per sample and a Q30 score of 85% or greater. A negative control sample was processed and sequenced in parallel in each sequencing run for quality control [27].
Bioinformatic analysis
High-quality sequencing data were generated by removing low-quality and short (length < 35 bp) reads using fastp software [28]. Human host sequences were subtracted by mapping to human reference genome sequences (National Center for Biotechnology Information GRCh38 assembly) using the Burrows-Wheeler Aligner tool (BWA) [29]. After the removal of low-complexity reads, the remaining data were classified by alignment to curated microbial genome databases for viruses, bacteria, fungi, and parasites. We developed a set of criteria similar to the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/genome/) criteria for selecting the representative assembly for microorganisms. After each microorganism is quantified, it is important to remove the contamination from the reagents. To determine the list of background microorganisms, we classified microorganisms detected in at least 25% of the samples, including negative controls. The quantitative value of the abundance of each microorganism and the total amount of nucleic acid were tested for correlation. When the quantitative value of the abundance of this microorganism was negatively correlated with the total amount of nucleic acid in the sample, it was determined as the reagent-derived background organisms.Therefore, they were excluded from the report. The sequencing data list was analyzed in terms of stringently mapped read number (SMRN, representing a species specific sequence), genome coverage (%) and relative abundance (%).
Threshold criteria for interpretation of metagenomic analysis
The microbial list obtained from the above analysis process was compared with an in-house background database, which contains microorganisms appearing in more than 50% samples in the laboratory in the past three months. The suspected background microorganisms were removed from the microbial list. For different types of microbes, the thresholds were set as follows: (1) Extracellular bacteria/fungus (excluding Cryptococcus)/parasites: SMRN ≥ 30, ranked among the top 10 for bacteria, fungi, or parasites. Organisms detected in the negative control group or that were present in ≥ 25% of samples from the previous 30 days were excluded but only if the detected SMRN was ≥ tenfold than that in the negative control group or other organisms. In addition, organisms present in ≥ 75% of samples from the previous 30 days were excluded. (2) Intracellular bacteria (excluding Mycobacterium tuberculosis and Brucella)/ Cryptococcus: SMRN ≥ 10, ranked among the top 10 for bacteria or fungi. Pathogens detected in the negative control group or that were present in≥ 25% of samples from the previous 30 days were excluded but only if the detected SMRN was ≥ tenfold than that in the negative control group or other organisms. (3) Virus/Brucella: SMRN ≥ 3, Pathogens detected in the negative control group were excluded but only if the detected SMRN was ≥ tenfold than that in the negative control group. (4) Mycobacterium tuberculosis: SMRN ≥ 1 [26, 30].
qPCR assay of P. jirovecii for validation experiment
The sequence of the primer pairs used is listed as follows: PjF (5′ -GCACGTTGGCCTCGTTTAC-3′) and PjR (5′ -GATGAAGCTCACTTTCCGATGAC-3′). The primers used in this qPCR assay target a 157 bp fragment. The qPCR assay was performed on Applied Biosystems 7500 Fast PCR system. The final reaction volume of 25 µL contained 12.5 µL of TaqMan Universal Master Mix (InvitrogenTM), MgCl2 (1.5 mL), 0.4 mM concentration of each primer (1 mL), 0.2 mM probe (0.5 mL) and 8.5 µL of extracted DNA. Thermal cycling conditions were as follows: preheating at 95 °C for 10 min, amplification of 45 cycles including denaturation at 95 °C for 20 s, annealing and extension at 60 °C for 1 min. Positive, negative, and extraction controls were included in each run. The CT value for positive samples was recorded for each run.
Clinical data collection
Clinical parameters of each patient were acquired through review of electronic medical records. We recorded patient data regarding demographics, pediatric risk of mortality score (PRISM), pediatric critical illness score (PCIS), underlying diseases, the length of ICU stay, use of immunosuppressant, laboratory test results, antibiotic treatment response and 30 days mortality.
Statistical analysis
Statistical analysis was carried out by an online statistics tool (http://dxonline.deepwise.com/) and Graphpad prism. Continuous variables were presented as medians and interquartile ranges and categorical variables as counts and percentages. The Wilcoxon Test was used for comparing the differences of continuous variables between PCP and non-PCP group and the X2 test for categorical variables. Spearman correlation test was used for analyzing correlation between the stringently mapped read numbers (SMRNs) of P. jirovecii detected by mNGS and LDH, serum BDG levels, pediatric risk of mortality (PRISM) as well as pediatric critical illness score (PCIS). Sensitivity, specificity, likelihood ratio, Jouden index, positive predict value (PPV) and negative predict value (NPV) was calculated using the clinical composite diagnosis as the reference standard. Significance was fixed at P < 0.05.
Results
Clinical characteristics and laboratory findings
There were 77 patients with severe pneumonia in this study, including 17 patients with PCP and 60 without PCP. The median age (4.3 years vs. 3 years), sex composition, pediatric risk of mortality (PRISM) and pediatric critical illness score (PCIS) were similar between the PCP group and the non PCP group. Patients with PCP mainly had dyspnea (88.2%), fever (76.5%) and cough (41.2%). Obviously, the PCP group was more prone to dyspnea than the non-PCP group. Various immunosuppressive conditions occurred in both groups. Corticosteroid use (41.2%), hematological malignancies (41.3%), and solid tumors (41.4%) all ranked first in PCP patients.
Compared with the non-PCP group, ARDS, mechanical ventilation and indwelling urinary catheter were more common in PCP. Days of mechanical ventilation in PCP group were longer. Peripheral blood lymphocyte, a median count of 0.34X109/L, was significantly lower in PCP patients. Serum BDG and LDH were significantly higher in PCP patients, respectively (P < 0.05). Ground-glass opacity was significantly more frequent in PCP patients (P < 0.05). In 33 ARDS patients, 14 patients were diagnosed with PCP by mNGS (Table 1).
Table 1.
The basic clinical data of enrolled patients
| Characteristics (median[IQR] or n[%] | PCP patients (n = 17) | Non-PCP patients (n = 60) | P value |
|---|---|---|---|
| Age (years) | 4.50 (3.00–7.00) | 3.00 (1.00–8.00) | 0.164 |
| Male | 11.00 (64.70%) | 37.00 (61.70%) | 0.891 |
| PRISM | 12.50 (10.00–15.00) | 13.00 (10.00–17.50) | 0.992 |
| PCIS | 82.00 (79.50–90.00) | 87.00 (84.00–93.00) | 0.100 |
| Clinical symptoms | |||
| Fever | 13.00 (76.50%) | 42.00 (71.20%) | 0.903 |
| Cough | 7.00 (41.20%) | 17.00 (28.80%) | 0.334 |
| Dyspnea | 15.00 (88.20%) | 16.00 (27.10%) | 0.000* |
| Immunocompromised conditions | |||
| Use of corticosteroids | 7.00 (41.20%) | 25.00 (42.40%) | 0.930 |
| Use of immunosuppressive agents | 1.00 (5.90%) | 2.00 (3.40%) | 1.000 |
| Hematologic malignancies | 7.00 (41.20%) | 23.00 (38.30%) | 0.832 |
| Solid tumors | 7.00 (41.20%) | 19.00 (31.70%) | 0.464 |
| Rheumatic diseases | 2.00 (11.80%) | 0.00 (0.00%) | 0.067 |
| Chest CT images | |||
| Ground-glass opacity | 10.00 (58.80%) | 1.00 (1.90%) | 0.000* |
| Patchy shadowing | 11.00 (64.70%) | 36.00 (66.70%) | 0.882 |
| Interstitial patterns | 3.00 (17.60%) | 25.00 (46.30%) | 0.035* |
| Pleural effusion | 1.00 (5.90%) | 13.00 (24.10%) | 0.195 |
| Cystic changes | 1.00 (5.90%) | 0.00 (0.00%) | 0.539 |
| Indwelling gastric tube | 15.00 (88.20%) | 45.00 (76.30%) | 0.466 |
| Indwelling urinary catheter | 14.00 (82.40%) | 33.0 (55.90%) | 0.048* |
| Indwelling vein catheter | 15.00 (88.20%) | 48.00 (81.40%) | 0.766 |
| Mechanical ventilation | 16.00 (94.10%) | 34.00 (57.60%) | 0.005* |
| Days of mechanical ventilation | 7.00 (5.00–8.50) | 4.00 (0.00–9.00) | 0.038* |
| Serum BDG (ng/L) | 435.16 (152.21–600.00) | 48.04 (37.50–153.90) | 0.027* |
| LDH (U/L) | 799.00 (566.00–921.00) | 354.50 (236.25–712.50) | 0.002* |
| CRP (mg/L) | 57.32 (15.91–90.75) | 41.13 (10.86–125.735) | 0.601 |
| PCT (ng/mL) | 0.37 (0.22–0.52) | 0.71 (0.27–7.06) | 0.085 |
| White blood cells (* 109/L) | 4.20 (2.07–10.91) | 6.39 (0.615–11.78) | 0.971 |
| Neutrophils (*109/L) | 3.18 (1.15–8.66) | 2.59 (0.115–7.598) | 0.576 |
| Lymphocytes (*109/L) | 0.34 (0.1–0.86) | 1.05 (0.36–3.65) | 0.025* |
| CRRT | 2.00 (11.80%) | 5.00 (8.30%) | 1.000 |
| ARDS | 14.00 (82.40%) | 19.00 (31.70%) | 0.000* |
| LOS in hospital | 13.00 (9.00–16.00) | 18.50 (9.75–40.25) | 0.060 |
| LOS in PICU | 10.00 (8.00–14.00) | 11.50 (7.75–20.00) | 0.337 |
| 30 days-mortality | 2.00 (11.80%) | 10.00 (16.90%) | 0.889 |
IQR interquartile range, PRISM pediatric risk of mortality score, PCIS pediatric critical illness scoring, Serum BDG Serum (1,3)-β-D-glucan, CT computed tomography, LDH lactate dehydrogenase, CRP C-reactive protein, PCT procalcitonin, CRRT continuous renal replacement therapy, ARDS acute respiratory distress syndrome, LOS length of stay
*P < 0.05
Performance comparison between mNGS and other diagnostic methods
The Ct value of P. jirovecii qPCR and mNGS sequencing results of 17 PCP cases in this study were listed in Table 2. Our result showed that the Ct value of all the cases with PCP detected by mNGS was less than 40, suggesting that the detection of PCP by mNGS was reliable.
Table 2.
The Ct value of P. jirovecii qPCR and mNGS sequencing results of 17 cases in this study
| Case No. | Sample type | Specific reads of mNGS results (n) | Ct value |
|---|---|---|---|
| 1 | Blood | Pneumocystis jirovecii (712) | 33.63 |
| 2 | Blood | Pneumocystis jirovecii (27), Parvovirus B19 (121) | 32.54 |
| BALF | Pneumocystis jirovecii (284), Acinetobacter baumannii (527) | 32.54 | |
| 3 | BALF | Pneumocystis jirovecii (7854) | 31.13 |
| 4 | BALF | Pneumocystis jirovecii (1104) | 24.48 |
| 5 | BALF | Pneumocystis jirovecii (367) | 31.13 |
| 6 | BALF | Pneumocystis jirovecii (5029) | 34.03 |
| 7 | BALF | Pneumocystis jirovecii (109,593), CMV (42), Aspergillus fumigatus (833), EB (18) | 27.75 |
| 8 | BALF | Pneumocystis jirovecii (487) | 35.39 |
| 9 | BALF | Pneumocystis jirovecii (9550), TTV (60), Staphylococcus epidermidis (18) | 31.80 |
| 10 | BALF | Pneumocystis jirovecii (1435) | 32.71 |
| 11 | Blood | Pneumocystis jirovecii (79), Streptococcus pneumoniae (27), Escherichia coli (53) | 32.15 |
| 12 | Blood | Pneumocystis jirovecii (6), Pseudomonas aeruginosa (83), Acinetobacter baumannii (359) | 35.25 |
| 13 | BALF | Pneumocystis jirovecii (871), parvovirus B19 (62) | 33.12 |
| Blood | Pneumocystis jirovecii (187), Parvovirus B19 (161,506) | 35.87 | |
| 14 | BALF | Pneumocystis jirovecii (22,576), Streptococcus pneumoniae (168) | 30.12 |
| 15 | BALF | Pneumocystis jirovecii (27,969) | 28.00 |
| 16 | Blood | Pneumocystis jirovecii (3024) | 34.78 |
| 17 | Blood | Pneumocystis jirovecii (1594), Corynebacterium matruchotii (106), CMV (72) | 32.00 |
The diagnostic performance of serum BDG and mNGS were compared in 17 PCP patients in our study. As illustrated in Table 3, blood and/or BALF from all patients were conducted by mNGS. The sensitivity and specificity of mNGS was 100% and 96.7%, which was remarkably higher than serum BDG (86.7% and 56.7%) and LDH (55.6% and 71.4%). The sensitivity of mNGS was higher than GMS staining (5.8%). We also found that PPV (89.5%) and NPV (100%) of the mNGS overtook that of serum BDG and LDH.
Table 3.
Diagnostic performance of mNGS, GMS staining, serum LDH and BDG for PCP
| Methods | PC group | Non-PCP group | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| mNGS | ||||||
| + | 17 | 2 | 100.0% | 96.7% | 89.5% | 100.0% |
| − | 0 | 58 | (77.1–100.0) | (87.5–99.4) | (65.5–98.2) | (92.3–100.0) |
| Serum BDG | ||||||
| + | 13 | 13 | 86.7% | 56.7% | 50.0% | 89.5% |
| − | 2 | 17 | (58.4–97.7) | (37.7–74.0) | (30.4–69.6) | (65.5–98.2) |
| GMS | ||||||
| + | 1 | 0 | 5.9% | 100.0% | 100.0% | 11.1% |
| − | 16 | 2 | (0.3–30.8) | (19.8–100) | (5.5–100) | (1.9–36.1) |
| LDH [30] | ||||||
| ≥ 618 U/L | 10 | 16 | 55.6% | 71.4% | 38.5% | 83.3% |
| < 618 U/L | 8 | 40 | (31.4–77.6) | (57.6–82.3) | (20.9–59.3) | (69.2–92.0) |
GMS staining gomori methenamine silver staining, serum BDG serum (1,3)-β-D-glucan, LDH lactate dehydrogenase, serum BDG < 80 ng/L was defifined as positive, LDH < 618 U/Lwas defifined as positive, CI confifidence intervals, PPV positive predict value, NPV negative predict value
mNGS for detection of P. jirovecii in blood and/or BALF samples
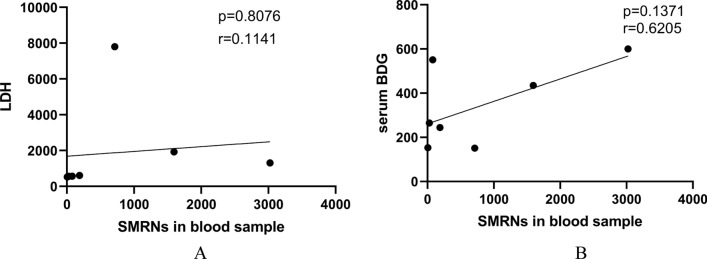
In total, there were 12 BALF samples and 7 blood samples in the patients with PCP (Table 4). Compared with survivors, non-survivors had higher read of SMRNs in BALF (P < 0.05). It is worth noting that the SMRNs in the BALF were significantly higher than that in blood samples (Fig. 2, 3232.00vs. 187.00, P = 0.022). Besides, the SMRNs of P. jirovecii detected by mNGS has a positive trend with serum BDG in blood (Fig. 3, R = 0.62, P > 0.05). Furthermore, we found non-survivors' lengths of stay in PICU were longer, but PRISM and PCIS in non-survivors were similar with survivors (Table 5). There was no correlation between the SMRNs of P. jirovecii and PRISM, PCIS as well as LDH.
Table 4.
mNGS for detection of P. jirovecii in blood and/or BALF Samples from PCP group
| Specimen | Patient nubmer | Positive | Negative |
|---|---|---|---|
| BALF only | 10 | 10 | 0 |
| Blood only | 5 | 5 | 0 |
| Both | 2 | 2 | 0 |
Fig. 2.
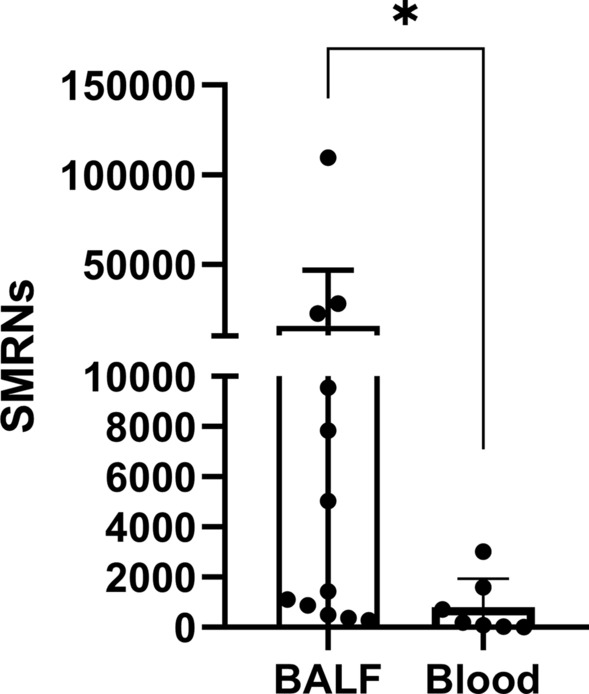
Comparison of the SMRNs of P. jirovecii detected by mNGS between BALF samples and blood samples
Fig. 3.
Correlation between the SMRNs of P. jirovecii detected by mNGS and serum LDH levels as well as serum BDG level
Table 5.
The wilcoxon test and the chi-square test analysis of risk factors for 30-days mortality
| Characteristics (median[IQR] or n[%] | Non-survivors (n = 2) | Survivors (n = 15) | P value |
|---|---|---|---|
| Age (years) | 9.50 (7.25–11.75) | 4.00 (3.00–7.00) | 0.204 |
| Male | 0.00 (0.00%) | 11.00 (73.30%) | 0.110 |
| PRISM | 13.50 (12.75–14.25) | 12.50 (10.00–15.00) | 0.808 |
| PCIS | 83.00 (78.50–87.50) | 82.00 (80.00–89.50) | 1.000 |
| Immunocompromised conditions | |||
| Use of corticosteroids | 1.00 (50.00%) | 6.00 (40.00%) | 1.000 |
| Use of immunosuppressive agents | 1.00 (50.00%) | 0.00 (0.00%) | 0.118 |
| Hematologic malignancies | 1.00 (50.00%) | 6.00 (40.00%) | 1.000 |
| Solid tumors | 1.00 (50.00%) | 6.00 (40.00%) | 1.000 |
| Rheumatic diseases | 0.00 (0.00%) | 2.00 (13.30%) | 1.000 |
| Indwelling gastric tube | 1.00 (50.00%) | 14.00 (93.30%) | 0.228 |
| Indwelling urinary catheter | 1.00 (50.00%) | 13.00 (86.70%) | 0.331 |
| Indwelling vein catheter | 2.00 (100.00%) | 13.00 (86.70%) | 1.000 |
| LDH (U/L) | 905.50 (901.75–909.25) | 630.00 (547.00–968.50) | 0.618 |
| CRP (mg/L) | 119.11 (67.51–170.70) | 57.32 (21.04–82.19) | 0.824 |
| PCT (ng/ml) | 1.05 (0.58–1.53) | 0.37 (0.23–0.49) | 1.000 |
| White blood cells (X 109/L) | 8.13 (6.07–10.18) | 4.20 (1.84–10.64) | 0.529 |
| Neutrophils (X 109/L) | 6.04 (3.45–8.64) | 3.18 (1.25–7.46) | 0.824 |
| Lymphocytes (X 109/L) | 0.56 (0.42–0.70) | 0.34 (0.09–1.17) | 0.824 |
| SMRNs in BALF | 68,781.00 (48,375.00–89,187.00) | 1269.50 (583.00–7147.75) | 0.030* |
| ARDS | 14.0 (82.4%) | 12 (80.0%) | 1.000 |
| Mechanical ventilation | 2.00 (100.00%) | 14.00 (93.30%) | 1.000 |
| Days of mechanical ventilation | 9.50 (8.25–10.75) | 7.00 (5.00–8.00) | 0.471 |
| LOS | 16.00 (15.50–16.50) | 13.00 (8.50–15.50) | 0.296 |
| LOS in PICU | 16.00 (15.50–16.50) | 10.00 (7.00–13.00) | 0.050* |
IQR interquartile range, PRISM pediatric risk of mortality score, PCIS pediatric critical illness scoring, Serum BDG serum (1,3)-β-D-glucan, LDH lactate dehydrogenase, CRP C-reactive protein, PCT procalcitonin, CRRT continuous renal replacement therapy, ARDS acute respiratory distress syndrome, LOS length of stay
*P < 0.05
Mixed infections and/or co-pathogens detected by mNGS
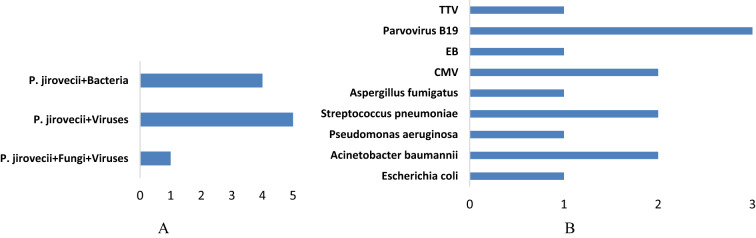
There are mixed infections and co-pathogens in 10 PJP patients identified by mNGS, including Acinetobacter baumannii, Streptococcus pneumoniae, Aspergillus fumigatus, Pseudomonas aeruginosa, Escherichia coli, Transfusion-transmitted virus, Parvovirus B19, Epstein-barr virus and Cytomegalovirus (Fig. 4). The respiratory pathogen detection results of mNGS and conventional methods are shown in Additional file 2: Table S1. The positive detection rate of various pathogens was higher using mNGS method than using conventional methods in both groups (Additional file 2: Table S2). The contents of pathogens in both groups by mNGS are shown in Additional file 2: Table S3. Only 41.2% of the observation group patients were with simple P. jirovecii infection while most manifested a mixed infection of P. jirovecii with viruses (29.4%), especially for CMV and parvovirus B19 (Additional file 2: Table S4).
Fig. 4.
Mixed infections and co-pathogens identified by mNGS in 17 PCP patients. A number of PCP patients with mixed infections; (B) number of PCP patients infected with various co-pathogens
Impact of mNGS on PCP patients’ antimicrobial therapy
According to the mNGS, 88.2% of the PCP critically pediatric patients modified their initial antimicrobial therapy. Trimethoprim-sulfamethoxazole was not received in 82.4% of patients until the report of mNGS results. There were 47.0% of PCP patients removing antimicrobial agents, 23.5% reducing antimicrobial spectrum and 29.4% adding antimicrobial agents (Table 6). Five cases diagnosed by mNGS were effectively treated with anti-PCP and discharged.
Table 6.
Impact of mNGS on PCP patients’ antimicrobial therapy
| Modififications(n [%]) | PCP patients (n = 17) % |
|---|---|
| Remove agent | 8(47.0) |
| Reduce antimicrobial spectrum | 4(23.5) |
| Add agent | 5(29.4) |
| Add TMP-SMZ | 14(82.4) |
| Add caspofungin | 1(5.9) |
| No change | 2(11.8) |
Discussion
PCP is a life-threatening opportunistic infection and an important cause of pneumonia in immunocompromised children [31, 32]. In fact, nowadays, PCP has a fair proportion in the non-HIV immunocompromised children. The rapid detection of pathogens by mNGS is conducive to the timely diagnosis and treatment of critically pediatric patients [33–35]. In this retrospective study of 17 PCP critically pediatric patients, the dominant underlying conditions included hematologic malignancies, solid tumors, and rheumatic diseases. Similar with previous researches, leukomonocyte of PCP patients was reduced, while serum LDH and BDG were typically elevated compared to the non-PCP patients. In addition, PCP patients usually suffer from mixed infection, and the hospital mortality rate reaches 11.8%.
The mNGS technology has the advantages of unbiased sequencing by extracting total DNA or RNA (usually subsequently reverse transcribed to DNA), fragmentation, library preparation and deep sequencing from original samples. As a new pathogenic gene detection and diagnosis technology, mNGS has the advantages of rapid, comprehensive and high sensitivity in the diagnosis of PCP. For patients with impaired immune function, the probability of mixed infections of multiple pathogens in the lungs is significantly increased. Using mNGS technology to detect pathogenic microorganisms in respiratory specimens of such patients can significantly improve the sensitivity (100%), specificity (96.3%) and time efficiency of diagnosis [13]. In this study, mNGS also showed an outstanding sensitivity and specificity in diagnosing PCP, consistent with other studies [37–42].In addition, mNGS facilitates unbiased identification of mixed infections through a single experiment. Previous studies have shown that mixed infections are common in PCP [43]. Coinfections of P. jirovecii are considered as index of poor prognosis [44]. In our study, about 59% of PCP patients had mixed infections, and the most common mixed infections were virus and bacteria. The detection rate of total and mixed pathogens was significantly higher than that of traditional pathogen detection. Notably, the coinfection of PCP with Parvovirus B19 is rarely reported. Parvovirus B19 is a small non-enveloped single-stranded DNA virus of the family Parvoviridae [47, 48]. Parvovirus B19 is pathogenic in human and causes a variety of clinical illnesses, including haematological diseases [49, 50]. In this study, Parvovirus B19 was detected from a patient with T-lymphoblastic lymphoma. The other patient is with acute B lymphoblastic leukemia. Whether children with blood diseases are more likely to be infected with PCP and Parvovirus B19 requires more cases support. Moreover, with 100% of PPV, mNGS results showed that there were 50% ARDS patients infected by P. jirovecii, which suggested early application of mNGS was beneficial to the rapid and proper diagnosis as well as precise treatment.
In our study, both blood and BALF samples were tested positive for P. jirovecii at the same time. It is widely reported that the pathogens from BALF were highly consistent with that from blood samples detected by mNGS Among that, we found the SMRNs in the BALF samples were significantly higher than that in blood samples, which suggested BALF samples were easier to detect pathogens in pneumonia. Blood samples may be a good alternative to BALF when bronchoscopic examination was infeasible.
In the present study, compared with survivors, non-survivors had higher reads of SMRNs in BALF. It is found that concurrent pathogen load correlates closely with the severity of sepsis and the survival rate of the ICU sepsis patients. It is reported that there was a positive correlation between the SMRNs of P. jirovecii with serum BDG both in blood and BALF samples. In other words, SMRNs may play a role in the severity of response. There was no correlation between the SMRNs of P. jirovecii and PRISM, PCIS, LDH as well as serum BDG in the present study. It is reported that the abundance of P. jirovecii in the blood of children is correlated with white blood cell counts and immune status [54]. It suggested that the SMRNs of P. jirovecii may be related to basal status of the patient. given that the sample size of our study was small, so that the correlation with SMRNs needs further study.Given that the sample size of our study was small, so that the correlation with SMRNs needs further study.
The unbiased broad-spectrum detection of mNGS could further provide guidance for valuable antimicrobial therapy. Based on the mNGS, 88.2% of the PCP critically pediatric patients modified their initial antimicrobial therapy and simplified the use of antibiotics. Trimethoprim-sulfamethoxazole was not received in 82.4% of patients until the report of mNGS results. Our data and previous studies showed that mNGS was excellent for the early and precise treatment of PCP. However, blind treatment based on mNGS alone is inappropriate, since mNGS technology cannot distinguish pathogens between colonization and infection. At the same time, due to its high sensitivity, false positive results may occur, while incomplete wall breaking may lead to false negative results. To our acknowledge, the occurrence and progress of infectious diseases involve the immune response of pathogens and hosts. Host response based detection has become an effective auxiliary means of traditional pathogen detection, which may improve the accuracy and efficiency of diagnosis. The combined application of host based detection and pathogen based detection is a new field worth exploring.
There were several limitations in this study. First of all, this study did not compare diagnostic performances of mNGS with PCR because PCR was not carried out routinely in our laboratory, which would be further explored in future work. Second, it was a retrospective and single-center research. In addition, the sample size of this study was small and bias was unavoidable. The diagnostic advantages of mNGS are obvious. Building a PCP diagnostic model based on mNGS and combining the characteristics of biomarkers of pathogen and host immune response will have important clinical value and potential application prospects, and will make precision treatment possible.
Conclusion
The mNGS technology is an efficient and useful diagnostic technology for PCP in critically pediatric patients. Both blood and BALF samples for mNGS are recommended for the presumptive diagnosis of PCP. SMRNs may be relevant to the prognosis, which needs further investigation.
Supplementary Information
Additional file 1: Figure S1. qPCR amplification curve and Ct value of P. jirovecii for mNGS validation in 5 cases.
Additional file 2: Table S1. The detected pthogens by mNGS and conventional clinical methods. Table S2. Diagnostic performance of mNGS, GMS staining,serum BDG and LDH in non-HIV-infected PJP patients. Table S3. The number of pathogens of different types by mNGS and conventional clinical methods. Table S4. The content of pathogens detected by mNGS in the observation group. Table S5. The qPCR and mNGS results of 17 cases in this study.
Author contributions
PC and YC contributed substance to ideas and design. HC and YC drafted the paper. HC, RW, YW, XZ, HH, XY and MH conducted statistical analysis on the data. JY was responsible for the mNGS result interpretation. PC, YL and YC gave a lot of assistance and revises manuscript. All authors contributed to the article and approved the submitted version. All authors read and approved the final manuscript.
Funding
This study was supported by Medical Scientific Research Foundation of Guangdong Province of China (A2021327).
Availability of data and materials
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board and Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University and conducted according to the Declaration of Helsinki. The ethics committee approved the waiver of informed consent owing to the retrospective nature of the review. All research data were de-identified and anonymously analyzed.
Consent for publication
Not applicable.
Competing interests
The authors declared that they had no competing financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hengxin Chen and Yujian Liang are contributed equally to this work
Contributor Information
Peisong Chen, Email: chps@mail3.sysu.edu.cn.
Yili Chen, Email: chenyli3@mail.sysu.edu.cn.
References
- 1.Chen X, Ding S, Lei C, et al. Blood and bronchoalveolar lavage fluid metagenomic next-generation sequencing in pneumonia. Can J Infect Dis Med Microbiol. 2020;2020:6839103. doi: 10.1155/2020/6839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan G, Liu J, Chen W, et al. Metagenomic next-generation sequencing of bloodstream microbial cell-free nucleic acid in children with suspected sepsis in pediatric intensive care unit. Front Cell Infect Microbiol. 2021;11:665226. doi: 10.3389/fcimb.2021.665226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cillóniz C, Dominedò C, Álvarez-Martínez MJ, et al. Pneumocystis pneumonia in the twenty-first century: HIV-infected versus HIV-uninfected patients. Expert Rev Anti Infect Ther. 2019;17(10):787–801. doi: 10.1080/14787210.2019.1671823. [DOI] [PubMed] [Google Scholar]
- 4.Saltzman RW, Albin S, Russo P, Sullivan KE. Clinical conditions associated with PCP in children. Pediatr Pulmonol. 2012;47(5):510–516. doi: 10.1002/ppul.21577. [DOI] [PubMed] [Google Scholar]
- 5.Cordonnier C, Alanio A, Cesaro S, et al. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients-authors' response. J Antimicrob Chemother. 2017;72(4):1266–1268. doi: 10.1093/jac/dkw580. [DOI] [PubMed] [Google Scholar]
- 6.Braga BP, Prieto-González S, Hernández-Rodríguez J. Pneumocystis jirovecii pneumonia prophylaxis in immunocompromised patients with systemic autoimmune diseases. Med Clin (Barc) 2019;152(12):502–507. doi: 10.1016/j.medcli.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Long S, Mu XD, Zhang C, et al. Epidemiological characteristics of pneumocystis Jirovecii infection and colonization in non-AIDS patients. Zhonghua Yi Xue Za Zhi. 2018;98(30):2414–2417. doi: 10.3760/cma.j.issn.0376-2491.2018.30.009. [DOI] [PubMed] [Google Scholar]
- 8.Ling C, Qian S, Wang Q, et al. Pneumocystis pneumonia in non-HIV children: a 10-year retrospective study. Clin Respir J. 2018;12(1):16–22. doi: 10.1111/crj.12467. [DOI] [PubMed] [Google Scholar]
- 9.White PL, Backx M, Barnes RA. Diagnosis and management of Pneumocystis Jirovecii infection. Expert Rev Anti Infect Ther. 2017;15(5):435–447. doi: 10.1080/14787210.2017.1305887. [DOI] [PubMed] [Google Scholar]
- 10.Nevez G, Chabé M, Rabodonirina M, et al. Nosocomial Pneumocystis Jirovecii infections. Parasite. 2008;15(3):359–365. doi: 10.1051/parasite/2008153359. [DOI] [PubMed] [Google Scholar]
- 11.Tang G, Tong S, Yuan X, et al. Using routine laboratory markers and immunological indicators for predicting Pneumocystis Jiroveci pneumonia in immunocompromised patients. Front Immunol. 2021;12:652383. doi: 10.3389/fimmu.2021.652383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahindra AK, Grossman SA. Pneumocystis Carinii pneumonia in HIV negative patients with primary brain tumors. J Neurooncol. 2003;63(3):263–270. doi: 10.1023/a:1024217527650. [DOI] [PubMed] [Google Scholar]
- 13.Tasaka S. Recent advances in the diagnosis and management of Pneumocystis pneumonia. Tuberc Respir Dis. 2020;83(2):132–140. doi: 10.4046/trd.2020.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, He T, Li X, Wang X, Peng L, Ma L. Metagenomic next-generation sequencing in diagnosis of a case of Pneumocystis Jirovecii pneumonia in a kidney transplant recipient and literature review. Infect Drug Resist. 2020;13:2829–2836. doi: 10.2147/IDR.S257587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Cissé OH, Kovacs JA. A molecular window into the biology and epidemiology of Pneumocystis spp. Clin Microbiol Rev. 2018;31(3):e00009–18. doi: 10.1128/CMR.00009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homayouni MM, Rostami A, Gholizadeh H, Mehbod ASA, Ebrahimi M, Mehravar S. Comparison of three cost effective staining methods for detection of Pneumocystis Jirovecii. J Parasit Dis. 2017;41(1):298–301. doi: 10.1007/s12639-016-0776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guegan H, Robert-Gangneux F. Molecular diagnosis of Pneumocystis pneumonia in immunocompromised patients. Curr Opin Infect Dis. 2019;32(4):314–321. doi: 10.1097/QCO.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 18.Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis Jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44(12b):1350–1363. doi: 10.1111/imj.12599. [DOI] [PubMed] [Google Scholar]
- 19.Asai N, Motojima S, Ohkuni Y, et al. Early diagnosis and treatment are crucial for the survival of Pneumocystis pneumonia patients without human immunodeficiency virus infection. J Infect Chemother. 2012;18(6):898–905. doi: 10.1007/s10156-012-0441-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ai JW, Cui P, Zhang WH, Wu HL, Ye MZ. A cluster of cases of Pneumocystis pneumonia identified by shotgun metagenomics approach. J Infect. 2019;78(2):158–169. doi: 10.1016/j.jinf.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Büchler AC, Lazarevic V, Gaïa N, et al. mycobacterium chelonae infection identified by metagenomic next-generation sequencing as the probable cause of acute contained rupture of a biological composite graft-a case report. Int J Mol Sci. 2021;23(1):381. doi: 10.3390/ijms23010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordey S, Brito F, Vu DL, et al. Astrovirus VA1 identified by next-generation sequencing in a nasopharyngeal specimen of a febrile Tanzanian child with acute respiratory disease of unknown etiology. Emerg Microbes Infect. 2016;5(7):e67. doi: 10.1038/emi.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Ai L, Zhou Y, Zhao Y, Huang J, Tang W, Liang Y. Rapid and precise diagnosis of pneumonia coinfected by Pneumocystis Jirovecii and Aspergillus fumigatus assisted by next-generation sequencing in a patient with systemic lupus erythematosus: a case report. Ann Clin Microbiol Antimicrob. 2021;20(1):47. doi: 10.1186/s12941-021-00448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Zhou J, Gui R, et al. Metagenomic next generation sequencing in the detection of pathogens in cerebrospinal fluid of patients after alternative donor transplantation: a feasibility analysis. Front Cell Infect Microbiol. 2021;11:720132. doi: 10.3389/fcimb.2021.720132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29(5):831–842. doi: 10.1101/gr.238170.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Durbin R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. 2010;26(5):589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Wang F, Zhang M, et al. Diagnostic value of bronchoalveolar lavage fluid metagenomic next-generation sequencing in Pneumocystis Jirovecii pneumonia in Non-HIV immunosuppressed patients. Front Cell Infect Microbiol. 2022;12:872813. doi: 10.3389/fcimb.2022.872813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakrzewska M, Roszkowska R, Zakrzewski M, Maciorkowska E. Pneumocystis pneumonia still a serious disease in children. J Mother Child. 2021;23(3):159–162. doi: 10.34763/devperiodmed.20192303.159162. [DOI] [PubMed] [Google Scholar]
- 32.Hughes WT, Price RA, Kim HK, Coburn TP, Grigsby D, Feldman S. Pneumocystis Carinii pneumonitis in children with malignancies. J Pediatr. 1973;82(3):404–415. doi: 10.1016/s0022-3476(73)80113-1. [DOI] [PubMed] [Google Scholar]
- 33.Yang A, Chen C, Hu Y, et al. Application of metagenomic next-generation sequencing (mNGS) using bronchoalveolar lavage fluid (BALF) in diagnosing pneumonia of children. Microbiol Spectr. 2022 doi: 10.1128/spectrum.01488-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin P, Chen Y, Su S, et al. Diagnostic value of metagenomic next-generation sequencing of bronchoalveolar lavage fluid for the diagnosis of suspected pneumonia in immunocompromised patients. BMC Infect Dis. 2022;22(1):416. doi: 10.1186/s12879-022-07381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graff K, Dominguez SR, Messacar K. Metagenomic next-generation sequencing for diagnosis of pediatric meningitis and encephalitis: a review. J Pediatric Infect Dis Soc. 2021 doi: 10.1093/jpids/piab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Bai L, Yang W, et al. Metagenomic next-generation sequencing for the diagnosis of Pneumocystis Jirovecii pneumonia in non-hiv-infected patients: a retrospective study. Infect Dis Ther. 2021;10(3):1733–1745. doi: 10.1007/s40121-021-00482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Y, Du J, Jin W, et al. Next generation sequencing for diagnosis of severe Pneumonia: China, 2010–2018. J Infect. 2019;78(2):158–169. doi: 10.1016/j.jinf.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Dubourg G, Raoult D. Emerging methodologies for pathogen identification in positive blood culture testing. Expert Rev Mol Diagn. 2016;16(1):97–111. doi: 10.1586/14737159.2016.1112274. [DOI] [PubMed] [Google Scholar]
- 39.Li D, Gai W, Zhang J, Cheng W, Cui N, Wang H. Metagenomic next-generation sequencing for the microbiological diagnosis of abdominal sepsis patients. Front Microbiol. 2022;13:816631. doi: 10.3389/fmicb.2022.816631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran PS, Wilson MR. Metagenomics for neurological infections—expanding our imagination. Nat Rev Neurol. 2020;16(10):547–556. doi: 10.1038/s41582-020-0374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thoendel MJ, Jeraldo PR, Greenwood-Quaintance KE, et al. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin Infect Dis. 2018;67(9):1333–1338. doi: 10.1093/cid/ciy303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrou J, Delastour SV. Predictive factors of Pneumocystis Pneumonia in patients with rheumatic diseases exposed to pro- longed high-dose glucocorticoids. Ann Rheum Dis. 2020;79(2):e23. doi: 10.1136/annrheumdis-2018-214718. [DOI] [PubMed] [Google Scholar]
- 43.Baumann S, Reinwald M, Haghi D, et al. Coinfection of Pneumocystis Jirovecii and invasive pulmonary aspergillosis in an immunocompromised patient: a diagnostic challenge. Onkologie. 2013;36:582–584. doi: 10.1159/000355168. [DOI] [PubMed] [Google Scholar]
- 44.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 45.Guo F, Kang L, Zhang L. mNGS for identifying pathogens in febrile neutropenic children with hematological diseases. Int J Infect Dis. 2022;116:85–90. doi: 10.1016/j.ijid.2021.12.335. [DOI] [PubMed] [Google Scholar]
- 46.Parize P, Muth E, Richaud C, et al. Untargeted next-generation sequencing-based fifirst-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. 2017 doi: 10.1016/j.cmi.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Cnc Garcia R, Leon LA. Human parvovirus B19: a review of clinical and epidemiological aspects in Brazil. Future Microbiol. 2021;16(1):37–50. doi: 10.2217/fmb-2020-0123. [DOI] [PubMed] [Google Scholar]
- 48.Gallinella G. The clinical use of parvovirus B19 assays: recent advances. Expert Rev Mol Diagn. 2018;18(9):821–832. doi: 10.1080/14737159.2018.1503537. [DOI] [PubMed] [Google Scholar]
- 49.Young NS. B19 parvovirus. Baillieres Clin Haematol. 1995;8(1):25–56. doi: 10.1016/s0950-3536(05)80231-8. [DOI] [PubMed] [Google Scholar]
- 50.Jain A, Jain P, Kumar A, Prakash S, Khan DN, Kant R. Incidence and progression of parvovirus B19 infection and molecular changes in circulating B19V strains in children with haematological malignancy: a follow up study. Infect Genet Evol. 2018;57:177–184. doi: 10.1016/j.meegid.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Zhao Y, Shang Y, et al. The clinical significance of simultaneous detection of pathogens from bronchoalveolar lavage fluid and blood samples by metagenomic next-generation sequencing in patients with severe pneumonia. J Med Microbiol. 2021 doi: 10.1099/jmm.0.001259. [DOI] [PubMed] [Google Scholar]
- 52.Duan LW, Qu JL, Wan J, et al. Effects of viral infection and microbial diversity on patients with sepsis: a retrospective study based on metagenomic next-generation sequencing. World J Emerg Med. 2021;12(1):29–35. doi: 10.5847/wjem.j.1920-8642.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Yuan M, Shi Y, Su X. Clinical performance of BAL metagenomic next-generation sequence and serum (1,3)-β-D-glucan for differential diagnosis of Pneumocystis Jirovecii pneumonia and Pneumocystis Jirovecii colonisation. Front Cell Infect Microbiol. 2021;11:784236. doi: 10.3389/fcimb.2021.784236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabodonirina M, Vaillant L, Taffé P, et al. Pneumocystis Jirovecii genotype associated with increased death rate of HIV-infected patients with pneumonia. Emerg Infect Dis. 2013;19(1):21–186. doi: 10.3201/eid1901.120140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue R, Wu X, Li T, Chang L, Huang X, Pan L. Early detection of Legionella pneumophila and Aspergillus by mNGS in a critically Ill patient with legionella pneumonia after extracorporeal membrane oxygenation treatment: case report and literature review. Front Med. 2021 doi: 10.3389/fmed.2021.686512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok CC, Lee KW, Ho CT, et al. A prospective study of survival and pro gnostic indicators of systemic lupus erythematosus in a southern Chinese population. Rheumatology. 2000;39(4):399–406. doi: 10.1093/rheumatology/39.4.399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. qPCR amplification curve and Ct value of P. jirovecii for mNGS validation in 5 cases.
Additional file 2: Table S1. The detected pthogens by mNGS and conventional clinical methods. Table S2. Diagnostic performance of mNGS, GMS staining,serum BDG and LDH in non-HIV-infected PJP patients. Table S3. The number of pathogens of different types by mNGS and conventional clinical methods. Table S4. The content of pathogens detected by mNGS in the observation group. Table S5. The qPCR and mNGS results of 17 cases in this study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.