FIGURE 1.
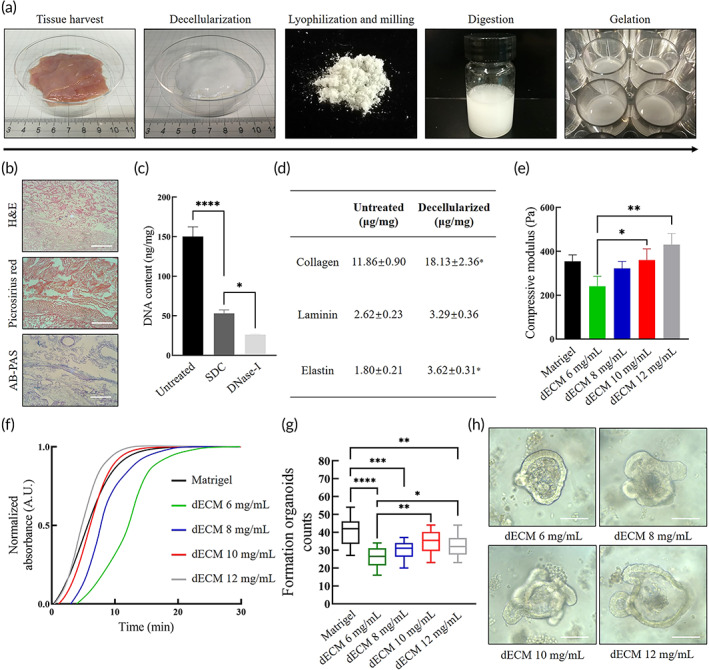
Characterization of decellularized extracellular matrix (dECM) hydrogel. (a) The preparation and gelation processes of dECM powder including harvesting of small intestine from freshly killed piglets, decellularization of the submucosa, lyophilization and milling into powder, sterilization, digestion in pepsin and HCl, adjustment of pH and salinity and incubation at 37°C. (b) Qualitative analysis of decellularized small intestine tissue by histological section including hematoxylin and eosin (H&E) for nucleus, Picrosirius Red (PR) for collagens and Alcian blue‐periodic acid‐Schiff (AB‐PAS) for glycosaminoglycans (GAGs). Scale bar 100 μm. (c) Quantitative analysis of DNA content in fresh untreated intestinal tissue, sodium deoxycholate (SDC)‐treated submucosa, and DNase‐treated submucosa. Mean ± SD (n = 3 batches). One‐way ANOVA. *p < 0.05 and ****p < 0.0001. (d) Quantitative ELISA analysis of dECM pregel including collagen, laminin, and elastin. Mean ± SD (n = 3 batches). Two‐sided t‐test *p < 0.05. (e) Compressive modulus measured by compressive testing. Mean ± S.D. (n = 3 samples). One‐way ANOVA. *p < 0.05 and **p < 0.01. (f) Turbidity analysis of dECM gels and Matrigel by spectrophotometry during heat‐mediated gelation. (g) Formed intestinal organoids per field of view at 100× at Day 7 at first passage. Mean ± S.D. (n = 16 from four organoids cultures). One‐way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. (h) Typical bright field images of formed organoids at Day 5 at first passage within dECM hydrogels. Scale bar 100 μm
