FIGURE 2.
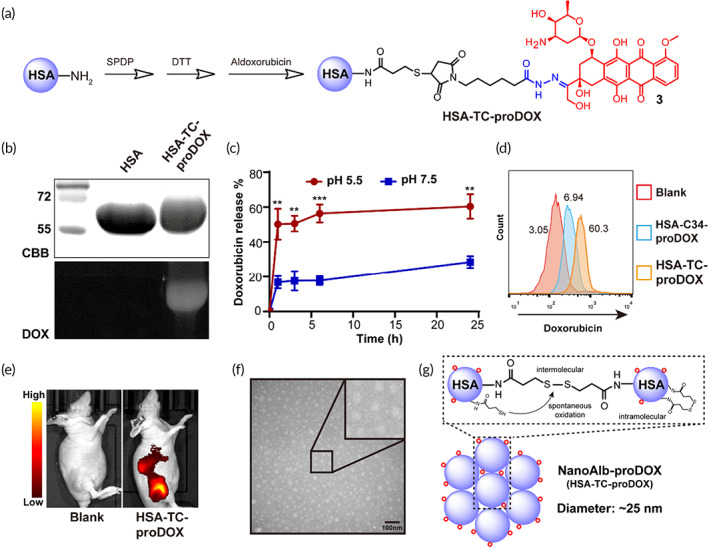
Synthesis and characterization of NanoAlb‐proDOX. (a) Schematic illustration of the synthesis of HSA‐TC‐proDOX. (b) SDS–PAGE analysis of HSA and HSA‐TC‐proDOX. In gel doxorubicin fluorescence of HSA‐TC‐proDOX indicated that HSA was successfully labeled with doxorubicin. CBB, Commassie brilliant blue staining; DOX, doxorubicin fluorescence. (c) pH‐dependent doxorubicin release from HSA‐TC‐proDOX. pH 7.5 and 4.5 were selected to simulate the environment of blood circulation or the tumor microenvironment, respectively. (d) Flow cytometry plots showing enhanced uptake of HSA‐TC‐proDOX by HeLa cancer cells. Conjugate with one micromolar doxorubicin was incubated with HeLa cells for 24 h and analyzed by flow cytometry. The percentages of positive cells are marked. (e) Accumulation of HSA‐TC‐proDOX in tumors. Cy5‐labeled HSA‐TC‐proDOX was intravenously injected into mice bearing MDA‐MB‐231 subcutaneous tumors, and fluorescence imaging of live animals was performed 24 h post‐injection. Accumulation of HSA‐TC‐proDOX in the tumor region was observed. (f) Transmission electron microscopy (TEM) image of HSA‐TC‐proDOX. Scale bar: 100 nm. (g) Proposed mechanism of the nanoparticle formation of HSA‐TC‐proDOX. The nanoparticle was speculated to assemble via the spontaneous oxidation of the excess sulfhydryl groups to form intermolecular disulfide bonds. Intracellular disulfide bonds and residual sulfhydryl groups may also exist. Red circles represent the proDOX molecules. HSA‐TC‐proDOX is hereafter named NanoAlb‐proDOX. Data are presented as the mean ± SEM. n = 3 technical replicates. **p < 0.01, ***p < 0.001
