FIGURE 9.
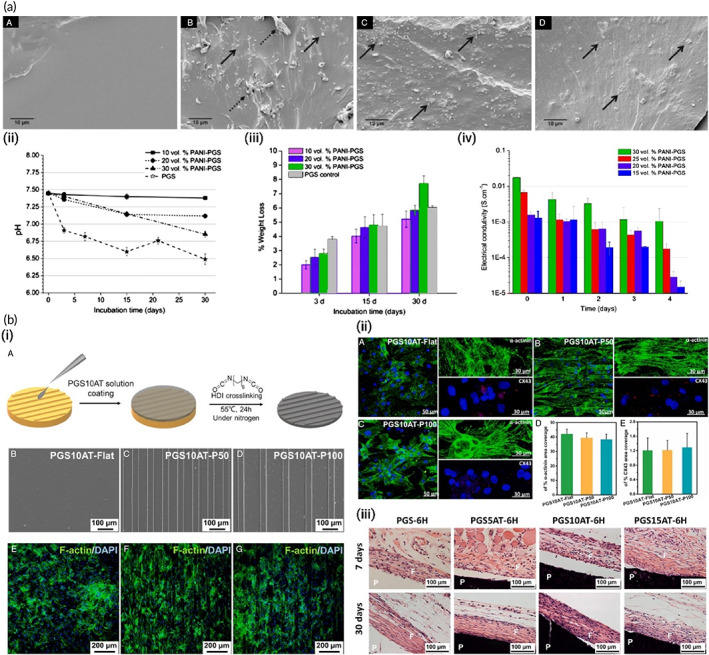
(a) Conductive polyaniline (PANI)–poly(glycerol sebacate) (PGS) composites for cardiac tissue engineering. (i) Representative SEM images showing cross‐section views of (A) pure PGS, (B) 10 vol% PANI–PGS, (C) 20 vol% PANI–PGS, and (D) 30 vol% PANI–PGS samples. Dashed arrows in (b) point to polymer matrix sheared during sample preparation. (ii) pH Variance in a 30 days period of in vitro degradation in PBS medium for pure PGS, and 10, 20, and 30 vol% PANI–PGS composites. (iii) Weight loss percentage of pure PGS, and 10, 20, and 30 vol% PANI–PGS composites due to in vitro degradation in PBS medium. (iv) Electrical conductivity alterations of 15, 20, 25, and 30 vol% PANI–PGS composites. Reproduced from Reference 147 with permission from Elsevier. (b) Micropatterned electroconductive poly(glycerol sebacate)‐aniline scaffolds for cardiac tissue engineering. (i) (A) schematic illustration of the preparation of micropatterned PGS10AT‐6H films. Representative SEM images of (B) PGS10AT‐Flat, (C) PGS10AT‐P50, and (D) PGS10AT‐P100 films. The immunofluorescence staining images show the F‐actin (green) and nuclei (blue) of CMs on (E) PGS10AT‐Flat, (F) PGS10AT‐P50, and (G) PGS10AT‐P100 films after culture for 2 days. (ii) The immunofluorescence staining images of the expression of cardiac‐specific markers (α‐actinin (green) and Cx‐43 (red)) on (A) PGS10AT‐Flat, (B) PGS10ATP50, and (C) PGS10AT‐P100 films after 8 days cultivation. (iii) Histological evaluation of subcutaneously implanted polymer films. Reproduced with permission from Reference 242, Elsevier
