FIGURE 13.
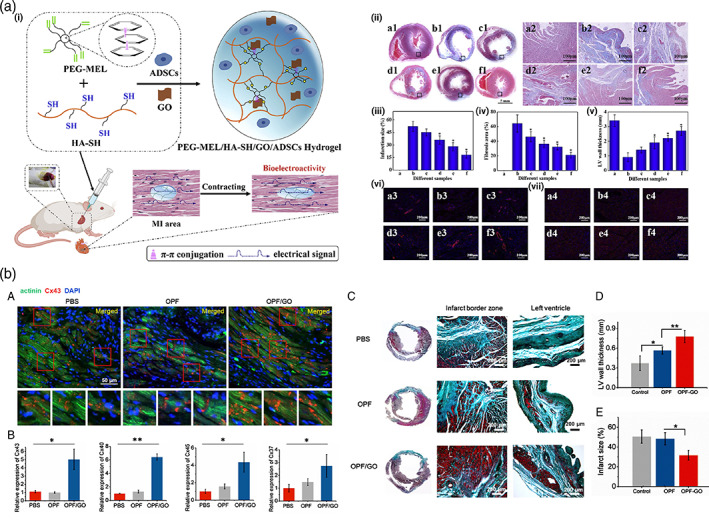
Graphene oxide application in cardiac tissue repair. (a) An injectable conductive hydrogel utilizing π‐π conjugation for cardiac tissue repair. (i) Schematic illustration of the application of soft and conductive PEG‐MEL/HA‐SH/GO hydrogel system for cardiac tissue repair. (A, A1–A4: Sham; B, B1–B4: PBS; C, C1–C4: PEG‐MEL/HA‐SH; D, D1–D4: PEG‐MEL/HA‐SH/GO; E, E1–E4: PEG‐MEL/HA‐SH/ADSCs; F, F1–F4: PEG‐MEL/HA‐SH/GO/ADSCs). (ii) Masson's trichrome staining for collagen (blue) and muscle (red); A2–F2 is a magnification of the corresponding black box labeled in A1–F1. (iii) Infarction size. (iv) Fibrosis area. (v) LV wall thickness. (vi) Immunofluorescence staining for a‐SMA. (vii) Immunofluorescence staining for Cx43. * shows a significant difference between the experimental group and PBS treated group. Reproduced from Reference 155 with permission from Elsevier. (b) Injectable oligo(poly(ethylene glycol) fumarate) (OPF)/graphene oxide hydrogels for cardiac tissue repair. (A) Immunofluorescence staining indicated increased gap junction remodeling in the infarcted region of the OPF/GO hydrogel‐treated group compared to the OPF‐ and PBS‐treated groups. (B) qPCR analysis of the expression levels of gap junction‐associated markers Cx43, Cx40, Cx37, and Cx45. (C) Masson trichrome staining of PBS‐, OPF‐, or OPF/GO‐injected heart 4 weeks after injection. (D) Left ventricle wall thickness and (E) infarct size measured for weeks post‐injection. Reproduced from Reference 311 under the terms of CC‐BY license open access
