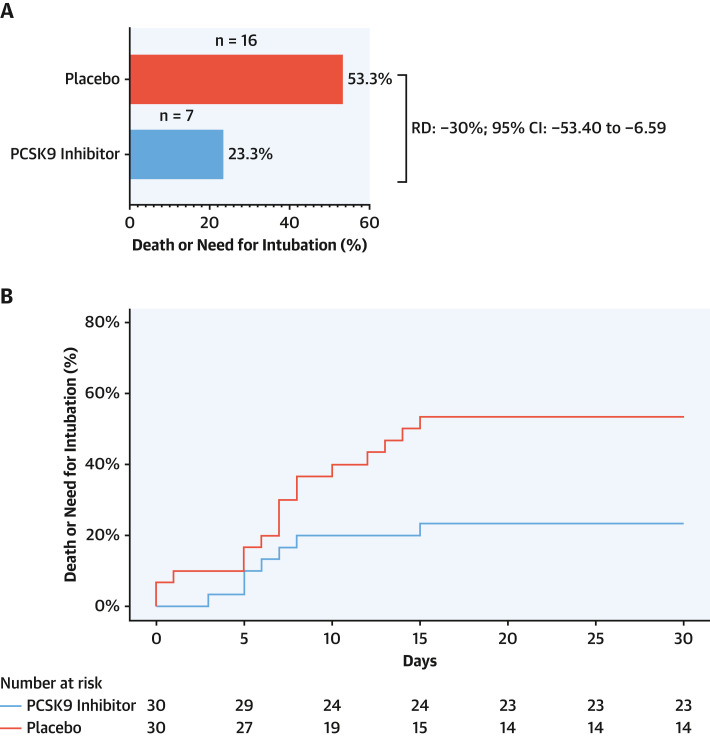
Figure 2.
Death or Need for Intubation
Death or need for intubation (primary endpoint) at 30 days among patients administered a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor or placebo. (A) Rates of primary endpoint in the PCSK9 inhibitor and placebo arms. (B) Cumulative incidence curves of 30-day death or need for intubation in the intention-to-treat population. n = number of events; RD = risk difference.