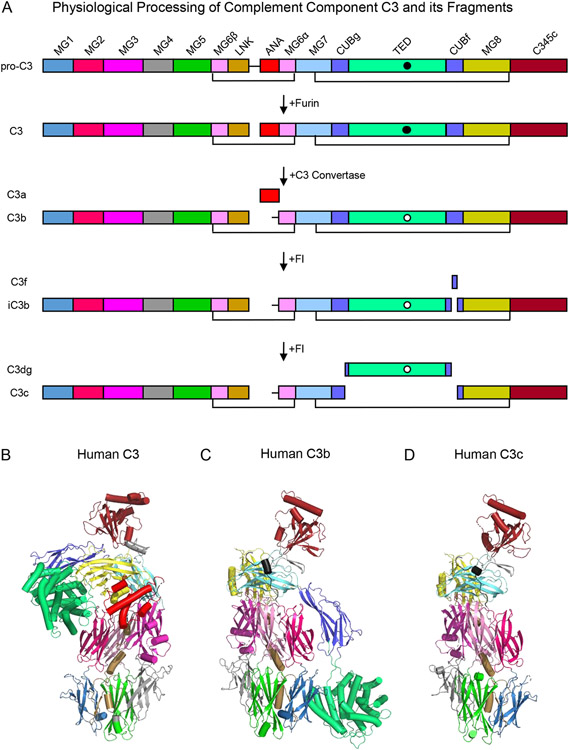
Figure 1.
Domain Organization and Three Dimensional Structures of Complement Component C3 and its Fragments. (A) Domain structure of human C3 and its fragments. The names of each fragment are shown at the left-hand side of the panel, while the names of each individual domain are presented atop the schematic that represents pro-C3. The identities of various proteases that catalyze processing of C3 are shown by the arrows that signify each transformation. Black lines represent key disulfide bonds that covalently link the C3 polypeptide chains together throughout proteolytic processing. The intramolecular thioester found in the TED domain is represented by a filled circle in its native state and by a clear circle in its hydrolyzed state. (B) Structure of native human C3 as drawn from the PDB entry 2A73 [12]. Individual domains are colored identically to panel A, with α-helices represented as cylinders and β-strands as arrows. (C) Structure of human C3b as drawn from the PDB entry 2I07 [15]. Structure of human C3c as drawn from the PDB entry 2A74 [12]. Note that the orientation of the structures shown in panels C and D are fixed about the so-called keyring core of the molecule comprised of domains MG1-MG6.