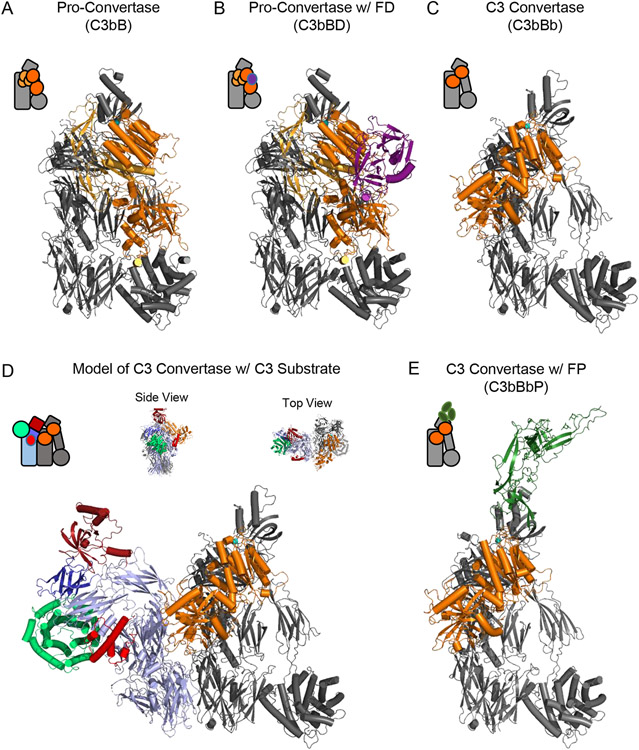
Figure 2.
Structural Features of the Alternative Pathway C3 Convertase. (A) Structure of the alternative pathway C3 pro-convertase as drawn from the PDB entry 2XWJ [24]. C3b is colored grey while FB is colored in shades of orange, with its Ba fragment in light orange and the Bb fragment in dark orange. A divalent cation, which in physiological conditions is Mg2+, is represented as a blue sphere. (B) Structure of the alternative pathway C3 pro-convertase bound to FD as drawn from the PDB entry 2XWB [24]. C3b and FB are colored as in panel A, while FD is colored purple. (C) Structure of the alternative pathway C3 convertase as drawn from the PDB entry 2WIN [33]. Proteins are colored identically to panel A. Note the loss of the Ba domain of FB that occurs following FD cleavage. The S. aureus immune evasion protein SCIN [31, 32, 92] is omitted from this image for clarity, although it was used to facilitate crystallization of this otherwise labile complex [33]. (D) Model of the alternative pathway C3 convertase bound to its native C3 substrate. C3b and Bb are colored identically to panel C, while native C3 appears with color scheme similar to that used in Figure 1. Both side and top-down views of the model are shown above the primary image. (E) Structure of the alternative pathway C3 convertase bound to a fragment of properdin as drawn from the PDB entry 6RUR [35]. C3b and Bb are colored identically to panel C, while the properdin fragment is colored green. The S. aureus immune evasion protein SCIN has likewise been omitted from this image for clarity. Simplified representations of each structure are shown in the top left corner of each panel.