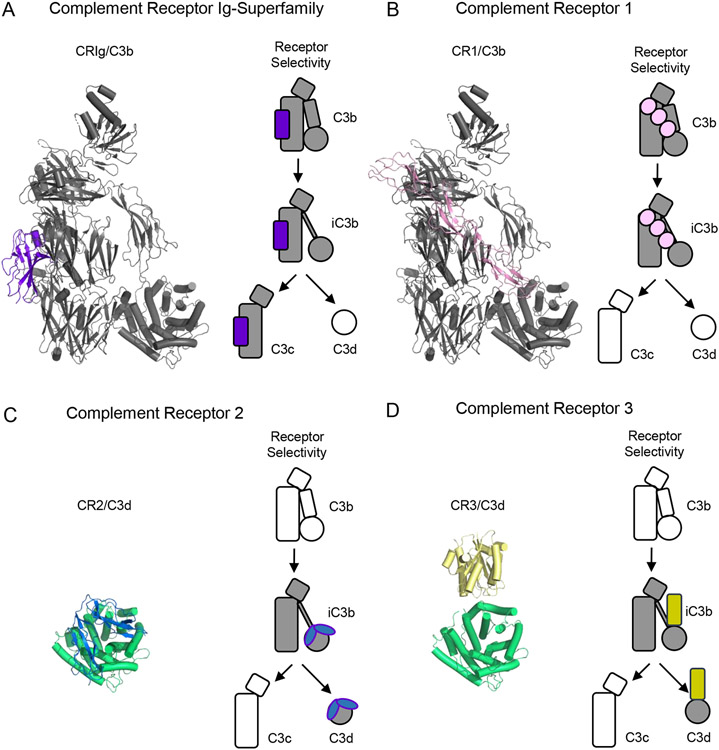
Figure 4.
Structural Features of Complement Receptors that Bind to C3-derived Opsonins and Fragments. (A) Structure of C3b bound to a domain from the Complement Receptor of the Ig-superfamily (CRIg) as drawn from PDB entry 2ICF [23]. C3b is colored grey, while CRIg is colored purple. (B) Structure of C3b bound to CCP domains 15-17 from Complement Receptor 1 (CR1) as drawn from PDB entry 5FO9 [43]. C3b is colored grey, while CR1 is colored pink. (C) Structure of CCP domains 1-2 from Complement Receptor 2 (CR2) bound to C3d as drawn from PDB entry 3OED [55]. C3d is colored green, while CR2 is colored blue. (D) Structure of the I-domain from Complement Receptor 3 (CR3) bound to C3d as drawn from PDB entry 4M76 [57]. C3d is colored green, while CR3 is colored yellow. Simplified representations of each receptor and the various fragments of C3 that it recognizes are shown at the right of each panel. Shaded images signify interactions known to occur, while hollow images signify activated forms of C3 that are not recognized by the receptor in question.