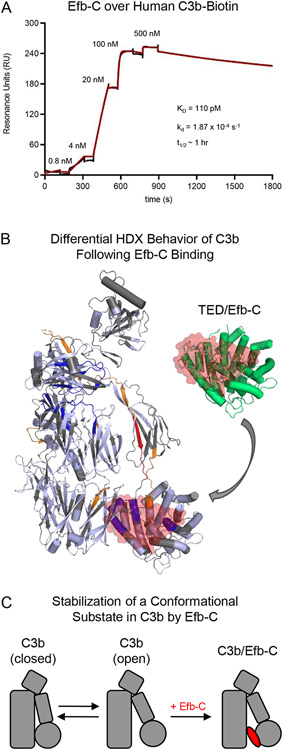
Figure 7.
Selective Stabilization of an Open Conformational State in C3b by the S. aureus Immune Evasion Protein Efb-C. (A) Characterization of Efb-C binding to site-specifically immobilized C3b as assessed by surface plasmon resonance. Human C3b that had been site-specifically biotinylated at its thioester cysteine [32, 66] was captured on a streptavidin-coated biosensor surface prior to injecting increasing concentrations of Efb-C in a single-cycle kinetic study. The reference-corrected sensorgram is shown with a black line while the outcome of fitting to a Langmuir model is shown with a red line. Efb-C binding to C3b exhibits low-picomolar affinity (KD ~110 pM) and is kinetically stable as indicated by its dissociation rate constant (10−4 s−1) (inset). (B) Differential Hydrogen/Deuterium exchange mapping of C3b as a function of Efb-C. The Hydrogen/Deuterium Exchange (HDX) profile was characterized by Mass Spectrometry for samples of C3b in the presence of either Efb-C or a non-binding mutant [76]. Peptides from C3b that exhibited increased HDX are colored either orange (moderate increase) or red (highest increase), while those that exhibited decreased HDX are colored dark blue. Peptides from C3b displayed no change are colored in pale blue, while peptides that were absent from the coverage map are colored grey. A full-scale representation of the structure of Efb-C bound to the TED domain from C3b [18] is inset at the top right of the panel, with Efb-C colored as a transparent red surface and TED colored green. Superposition of this structure onto the HDX map of C3b reveals that residues comprising the Efb-C binding site within the TED domain are highly exchange-protected, thereby demonstrating consistency between the crystallographic and in solution studies. (C) Simplified representation showing stabilization of an open conformational state in C3b upon Efb-C binding. As the interaction between Efb-C and C3b is ultra-high affinity and kinetically stable, Efb-C binding is depicted as an essentially irreversible step in this mechanism.