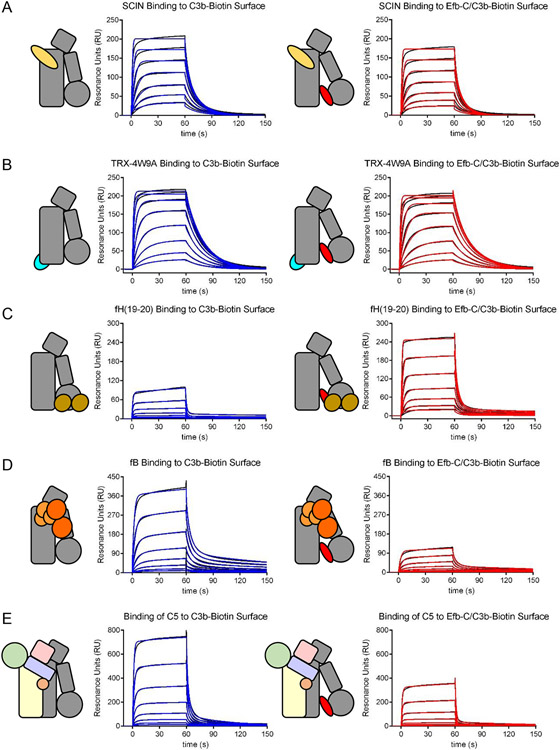
Figure 8.
Certain Ligands Exhibit Conformational Dependence in Their Affinity for C3b. The binding of various ligands to either C3b alone (left panels) or C3b that had been presaturated with Efb-C and therefore trapped in the open state (right panels) was assessed by surface plasmon resonance. Reference-corrected sensorgram series are shown with black lines, while the outcome of fitting to kinetic models is shown with either blue (C3b alone) or red (Efb-C/C3b) lines. (A) Comparison of binding of the S. aureus immune evasion protein SCIN. (B). Comparison of binding of a fusion protein consisting of E. coli thioredoxin and a minimal Compstatin sequence [82]. (C) Comparison of binding of CCP domains 19-20 of human FH. (D) Comparison of binding of human FB. (E) Comparison of binding of human C5. Whereas neither SCIN nor TRX-4W9A displayed sensitivity to the presence of Efb-C, FH(19-20) exhibited increased affinity for C3b in the presence of Efb-C, presumably due to increased exposure of its binding site within the TED domain. By contrast, the affinity of both FB and C5 were significantly diminished in the presence of Efb-C. This suggests that molecules which trap C3b in an open conformational state may be potent inhibitors of those aspects of complement function dependent upon FB/C3b and C5/C3b interactions.