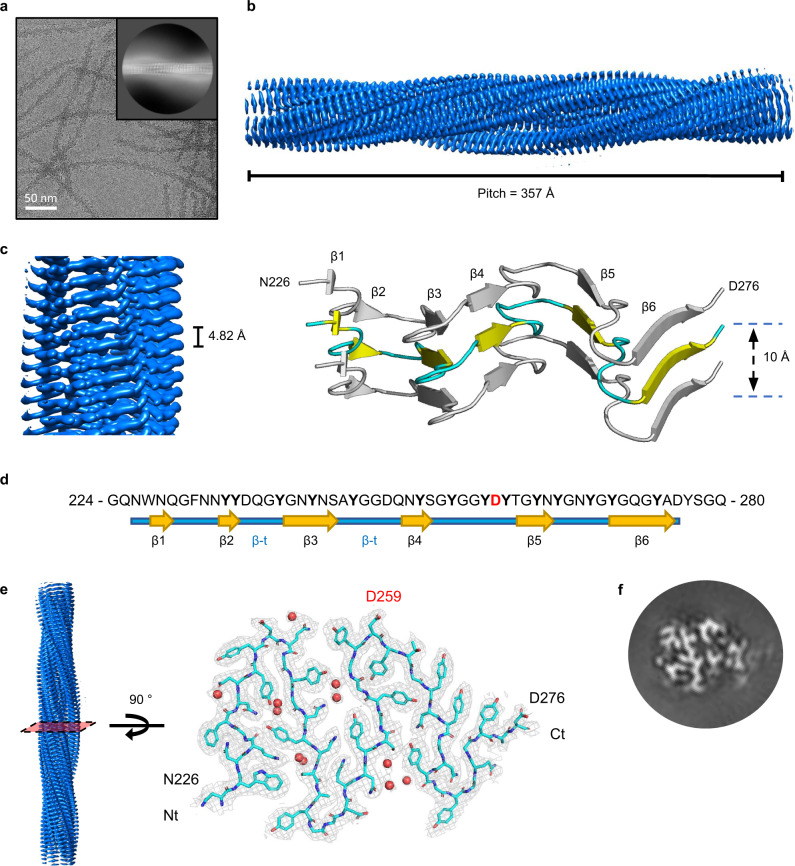
Fig. 2. Structure of hnRNPDL-2 amyloid filaments.
a Cryo-electron micrograph of hnRNPDL-2 filaments. Representative image from 1114 micrographs. The inset shows a representative reference-free 2D class average image of the hnRNPDL-2 filament. Scale bar, 50 nm. b Side view of the three-dimensional cryo-EM density map showing the structured core of an individual hnRNPDL-2 filament. The fibril pitch is indicated. c Detailed view of the cryo-EM density map showing the layer packing in the hnRNPDL-2 filament (left panel). The filament rise/subunit is indicated in Å. Rendered side view of the secondary structure elements accounting for three stacked rungs comprising residues N226-D276 (right panel). The distance between the stacks is indicated in Å. d Sequence of hnRNPDL exon 6, according to hnRNPDL-2 numbering. The observed β-strands that build the hnRNPDL-2 fibrils amyloid core are indicated. e Cryo-EM density map of a layer of hnRNPDL-2 amyloid core. Fitting of the atomic model for residues N226-D276 is shown on top. D259 disease-associated amino acid is highlighted in red. Water molecules are shown as red spheres. f Representative 3D class average image of the hnRNPDL-2 amyloid fibril.