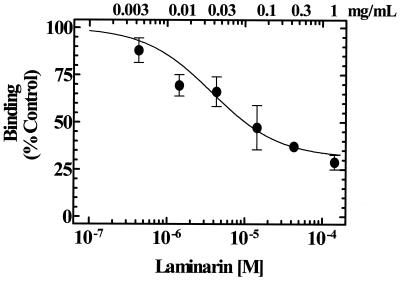
FIG. 3.
Laminarin competition for NHDF binding to immobilized DAP-glucan phosphate. The displacement relationship was characteristic of a single binding site with a KD of 3.7 μM (95% CI, 1.9 to 7.3 μM) and a maximum displacement of 69% ± 6%. The failure of laminarin to completely inhibit the interaction of NHDF and immobilized DAP-glucan phosphate suggests the presence of two types of binding interactions, one of which is not inhibitable by laminarin.