Abstract
Introduction
Neurological diseases, including Alzheimer's, Parkinson's diseases, and brain cancers, are reportedly caused by genetic aberration and cellular malfunction. Herbs with bioactive compounds that have anti-oxidant effects such as cordyceps and turmeric, are of interest to clinical applications due to their minimal adverse effects. The aim of study is to develop the nanoencapsulated cordyceps and turmeric extracts and investigate their capability to enhance the biological activity and improve neuronal function.
Methods
Human neuroblastoma SH-SY5Y cells were utilized as a neuronal model to investigate the properties of nanoencapsulated cordyceps or turmeric extracts, called CMP and TEP, respectively. SH-SY5Y cells were treated with either CMP or TEP and examined the biological consequences, including neuronal maturation and neuronal function.
Results
The results showed that both CMP and TEP improved cellular uptake efficiency within 6 h by 2.3 and 2.8 times, respectively. Besides, they were able to inhibit cellular proliferation of SH-SY5Y cells up to 153- and 218-fold changes, and increase the expression of mature neuronal markers (TUJ1, PAX6, and NESTIN). Upon the treatment of CMP and TEP, the expression of dopaminergic-specific genes (LMX1B, FOXA2, EN1, and NURR1), and the secretion level of dopamine were significantly improved up to 3.3-fold and 3.0-fold, respectively, while the expression of Alzheimer genes (PSEN1, PSEN2, and APP), and the secretion of amyloid precursor protein were significantly reduced by 32-fold and 108-fold, respectively. Importantly, the autophagy activity was upregulated by CMP and TEP at 6.3- and 5.5-fold changes, respectively.
Conclusions
This finding suggested that the nanoencapsulated cordyceps and turmeric extracts accelerated neuronal maturation and alleviated neuronal pathology in human neural cells. This paves the way for nanotechnology-driven drug delivery systems that could potentially be used as an alternative medicine in the future for neurological diseases.
Keywords: Cordyceps, Turmeric, Neuronal maturation, Nanoencapsulation, Autophagy
Introduction
Neurological disorders include a variety of hereditary and sporadic diseases, involving the chronic and progressive loss of neuronal structures and functions. The occurrence of symptoms depends on the part of the brain that is affected by the diseases.9 Alzheimer's disease (AD) is a result of specific brain cell damage, which is caused by the loss of neural connections within the brain. Parkinson's disease (PD) deteriorates dopaminergic neurons in the basal ganglia and the substantia nigra, and could result from genetic aberrations, in particular alpha-synuclein SNCA and Leucine-rich repeat kinase 2 gene (LRRK2).37 The therapeutic approaches for both Alzheimer's disease and Parkinson's disease are mostly palliative cares, and hardly improve the disease symptoms. For this reason, the AD/PD etiology becomes to interest to investigate the molecular mechanisms of the disease, and develop novel therapeutic options. Human neuroblastoma SH-SY5Y cells are widely used as a cellular model for studying neurotoxicity, brain cancer, and neurogenerative disorders.3–7
Cordyceps militaris, a medicinal mushroom, is now widely used in pharmaceutical and cosmeceutical industries.23 Several studies have demonstrated that Cordycepin is a key bioactive compound of Cordyceps militaris that attenuates oxidative stress, protects against cancer progression, and alleviates cells to normal function.7,13 Turmeric is another prominent medicinal herb, which could exert variety of health benefits.1 Curcumin (diferuloylmethane) is a phytopolyphenol compound isolated from turmeric, and possesses pharmacologic properties, such as anti-inflammatory, antioxidant, and anticancer. The modifications of cordyceps and turmeric extracts for relieving neurological conditions was focused in this study.
The focus of this study was on nerve cancer cells as it has been previously reported that the use of other cell types may be limited, e.g., primary rodent neurons derived from embryonic central nervous system tissue is. limited by the fact that they do not express the human proteins most closely associated with neurodegenerative diseases.20 The human neuroblastoma cell line SH-SY5Y is frequently used as an in vitro model for neurodegenerative disease studies. Because SH-SY5Y cells are derived from the sympathetic nervous system and considered to be derived from a neuronal lineage in its immature stage. In addition, SH-SY5Y neuroblastoma cells reveal a number of morphological and biochemical events. Including a decrease in the rate of proliferation Nerve cell formation and proliferation and the expression of maturing neuronal markers. This makes it phenotypically closer to that of primary neurons, and SH-SY5Y cells have human protein expression.33
Nanotechnology increases level of interest for the development of nanoparticle and nanoencapsulation that can be derived from proteins, biopolymers, or polysaccharides. Advantages of natural polymers are non-toxic, biodegradable, and biocompatible.29,30 Several approaches have proposed for herbal capsulation, including nanocomplexation, gelation, complex coacervation, Electrospray, and solvent-free pH-driven encapsulation.10 Nanoencapsulation obtained from starch received increasing attention nowadays, since they can be used to produce large quantities of nanoencapsulated products that are not harmful to human health.17 Nanoencapsulation from starch preparations, such as sago flour, corn, or cassava starch, prepared by the precipitation method, resulting in nano-size particles of approximately 300–600 nm.14–16
In this study, we are interested in using cassava starch to improve encapsulation of cordyceps extract and turmeric since cassava starch is a cheap plant and has a small particle size. The cordyceps extract-loaded cassava starch (CMP) and turmeric extract-loaded cassava starch (TEP) were applied to human neuroblastoma SH-SY5Y cells, and assessed their influences in neuronal functions. It was found that CMP/TEP could promote the maturation and attenuate AD/PD pathology of human neuroblastoma SH-SY5Y cells through autophagy activation. Therefore, the nanotechnology-driven drug delivery systems of cordyceps/turmeric extracts could improve neuro-promoting effects, and might be applied for treating neurological disorders in the future.
Material and Methods
Chemicals and Reagents
Cordyceps was manufactured from Laboratory of Cell-Based Assays and Innovations (CBAI), School of Biotechnology, Institute of Agricultural Technology, Suranaree University of Technology. Turmeric extracts were purchased from SAND-M Global Co., Ltd. The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Dulbecco's Modified Eagle Medium (DMEM), and fetal bovine serum (FBS) were purchased from HyClone (HyClone, Logan, UT). The cassava starch powder was purchase from E.T.C. Eaib Tong Chan Co., Ltd., Thailand. Human neuroblastoma (SH-SY5Y) cell lines were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA).
Fabrication And Characterization of Nanoencapsulated Cordyceps/Turmeric Extracts
The fabrication methods of CMP and TEP were performed by using 4% of hydrolysed starch from physical treatments, mixed with 100 ml of cordyceps extract (1,500 mg L−1 of cordycepin) or 1,000 mg of turmeric extract (296 mg L−1 of curcumin).17 The size distribution of CMP and TEP was used nanoparticle tracking analysis system by NanoSight NS300. The concentrations of cordycepin and curcumin in solutions were determined using high performance liquid chromatography (HPLC) and fluorescence microplate reader. The calibration curves were created with standard cordycepin and curcumin.
Cytotoxicity Assays
MTT cell survival assay was used to determine the effect of cordycepin, curcumin, cordyceps extract (CM), turmeric extract (TE), CMP, and TEP on cell viability. Briefly, human SH-SY5Y cells were seeded on 96-well plates at density of 1 × 104 cells/well. Cells were then treated with cordycepin, curcumin, cordyceps, CM, TE, CMP, and TEP in a various concentration for 24 h. Then, MTT solution was added to each well and incubated at 37 °C in the dark for 4 h, the formazan crystal was dissolved with DMSO and the formazan solution was the absorbance at 570 nm by using a microplate reader (BMG Labtech, Ortenberg, Germany). The 50% inhibitory concentration (IC50) was obtained from the dose–response curve of percent viability (Y) versus concentration tested (X), and calculated with a linear regression performed using Microsoft Excel.
Cell Morphology and Protein Secretion Assay
From the cytotoxicity results, we are selected the concentration for the concentrations for the cell morphology assay and the protein secretion assay. To determine the morphological change and the level of secretory protein of the treated cells, SH-SY5Y cells were treated with a various concentration of CMP (0.09, 0.37 and 1.46 µM), and TEP (0.09, 0.35 and 1.38 µM) for 24 h (data not shown) or 0.09 µM/day of CMP and TEP for 10 days. The cell morphology was examined using an inverted microscope, and determined the daily protein secretion levels using BCA assay. Briefly, the culture medium of neurons was collected 24 h after treatment and 10 μl of the sample was added to 96-well plates and 200 μl of the working medium was added into each well. The plates were then turned off and incubated at 37 °C for 30 min. The absorbance was measured at or near 562 nm on a plate reader. The secreted protein was calculated as cumulative secretion protein on days 5 and 10 post-treatment compared with protein secretion levels of the untreated cells at days 0 and 5.
Absorption Level and Cellular Uptake Assay
The absorption level of nanoencapsulated molecules was compared the percentage of cellular uptake with the native natural extract in human neuroblastoma SH-SY5Y cells. The culture cells were treated with 3 µM of CM, CMP, TE, and TEP, and assessed at different time points (5, 15, 30 min, and 1, 2, 4, 6 h). After the treatment, the supernatant was collected at different time points (5, 15, 30 min, and 1, 2, 4, 6 h) and measured the concentration of cordycepin by using HPLC. The relative fluorescence level of TE and TEP (Turmeric contains curcumin that can be a strong fluorescent agent.) within the neuroblastoma cell were evaluated with the lysed cells using 100% DMSO. Samples of 400 µl curcumin concentrations were measured by using fluorescence microplate reader and compared with standard curcumin. The percentage of cellular uptake was calculated using the initial concentration as 100% cellular uptake.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
To determine the expression of neuronal markers, dopaminergic neuron-specific and Alzheimer’s disease-related genes were assessed after the treatment. Briefly, human SH-SY5Y cells were seeded on 6-well plates at density of 2 × 105 cells/well. The cells were treated with 0.09 µM/day of CMP and TEP for 5 and 10 days, respectively. Thereafter, the total RNA was isolated using a NucleoSpin RNA Plus kit (Macherey–Nagel, Dueren Germany), according to the manufacturer’s protocol. Subsequently, 1 µg of RNA was used for complementary DNA (cDNA) synthesis by ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO CO., LTD., Japan). The PCR was carried out in a Biorad/C1000Touch thermocycler (Biorad, CA, USA) with specific primers, and amplified cDNA products were identified by electrophoresis using a 1.5% agarose gel. The gel was visualized using Ethidium bromide staining and gel documentation. The relative expression level of a target gene was quantified by normalization with Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) gene as an internal control. Primers used in the experiment were as follows in Supplementary Table S1.
Immunofluorescence and Monodansylcadaverine (MDC) Assay
For detecting neuron proteins, the cells were cultured in 24-well plates, and treated with 0.09 µM/day of either CMP or TEP for 5 days. The treated cells were then stained with the primary antibody (1:500 of rabbit anti-polyclonal beta tubulin antibody and mouse anti-PAX6 antibody) prior to applying the secondary antibody (1:500 of rhodamine conjugate goat anti-rabbit antibody and 1:1000 of Alexa fluor™ 488 conjugated goat anti-mouse antibody). For detecting autophagy-related protein, the treated cells were stained with 1:1000 of mouse anti-LC3 antibody, and then applied 1:1000 of Alexa fluor™ 488 conjugated goat anti-mouse antibody. For detecting autophagic vacuoles, the treated cells were stained with 50 µM of MDC for 20 min at room temperature (RT). The stained cells were observed under the fluorescence microscope.
Chemiluminescent Enzyme-Linked Immunosorbent Assay (ELISA)
To measure the secretion level of dopamine, the supernatant was collected from the neuronal cells, and determined by ELISA assay. For the secretion level, dopamine was determined by the competitive-ELISA assay using dopamine ELISA Kit (Elabscience, Wuhan, Hubei, China). Briefly, 50 µl of each sample and 1 × biotinylated detection antibody solution were loaded into the micro-ELISA plates that pre-coated with dopamine. The competing reaction was incubated at 37 °C for 45 min. After washing 2–3 times with washing buffer, horseradish peroxidase (HRP)-conjugated avidin solution was added 100 µl/well, and incubated at 37 °C for 30 min. Then, the TMB substrate solution was added, and the enzyme–substrate reaction was measured spectrophotometrically at a wavelength of 450 nm.
To measure the secretion level of human amyloid precursor protein (APP), the secretion level of APP was determined by sandwich-ELISA assay using human APP Duo Set ELISA (R&D Systems GmbH Wiesbaden, Germany). Briefly, 100 µl/well of mouse anti-human APP-capture antibody was coated onto the 96-well microplate overnight at room temperature. After blocking and washing, 100 µl of each sample was applied into the well, and incubated at room temperature for 2 h. The APP-capture antibody was incubated with 100 µl of biotinylated mouse anti-human APP detection antibody for 2 h and streptavidin conjugated HRP for 20 min. Then, peroxide and chromogen solution were added at ratio 1:1, and the enzyme–substrate reaction was measured spectrophotometrically at a wavelength of 450 nm.
Statistical Analysis
All experiments were performed in triplicates, and data were expressed as mean ± standard deviation.41 Statistical analysis was performed using SPSS (version 26.0, SPSS Inc., USA). Significant differences between the treatment and control were determined by one-way ANOVA analysis, followed by student T-tests, and *p < 0.05, **p < 0.01 and ***p < 0.001 was considered as statistically significant.
Results
Characterization of Nanoencapsulated CMP/TEP and Their Bioactivity
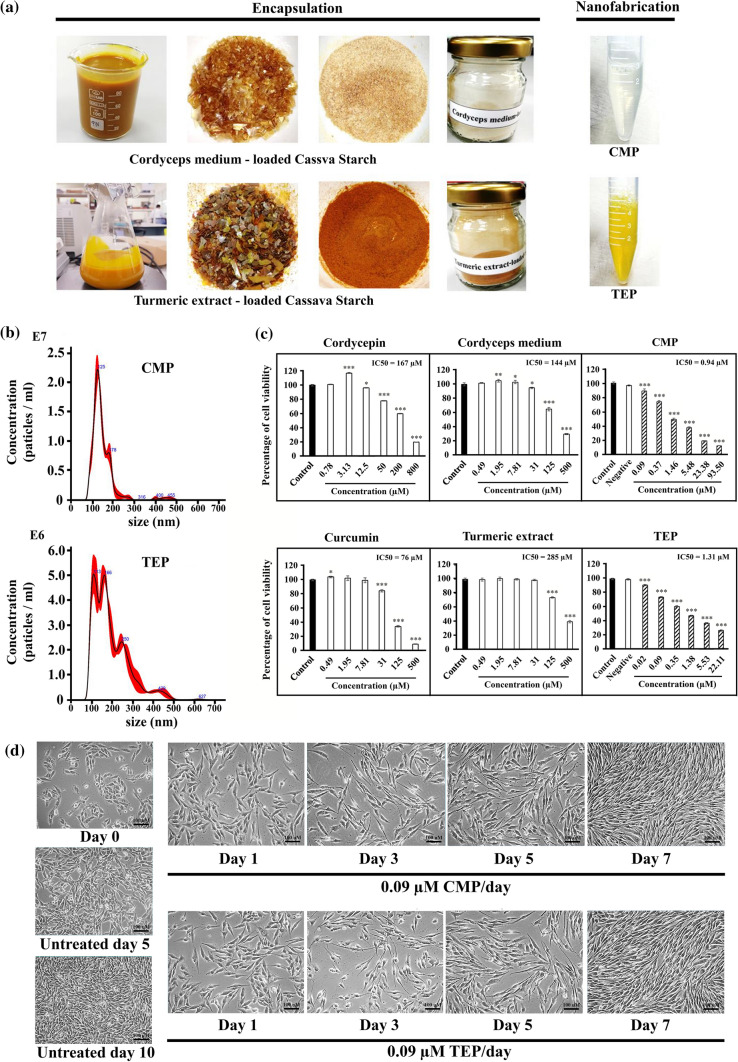
The fabrication of nanoencapsulation for cordyceps and turmeric extracts was shown in Fig. 1A. The CMP appeared as golden yellow crystals, while the TEP was orange. The size distribution and number of particles of CMP and TEP in nanosuspension (n = 5/group) were analysed by nanoparticle tracking analysis system using NanoSight NS300. The result showed that the average particle size of CMP and TEP was 146.1 ± 1.2 nm and 195.2 ± 6.1 nm, respectively (Table 1 and Fig. 1b). The result in this study was comparable to our previous work, representing the reproducible process.17 To evaluate the effects of CMP/TEP on cellular toxicity, cordycepin, curcumin, CM, TE, CMP, and TEP were applied to human neuroblastoma SH-SY5Y cells, and assessed by MTT colorimetric method. CMP improved the IC50 efficiency against SH-SY5Y cells up to 178- and 153-fold changes, compared with cordycepin and CM, respectively (Fig. 1c). Similarly, the nanoencapsulated TEP increased the anticancer efficiency against SH-SY5Y cells to 58- and 218-fold changes, compared with curcumin and TE, respectively (Fig. 1c). These results indicated that the CMP and TEP prominently enhanced their bioactivity to human neuroblastoma SH-SY5Y cells.
Figure 1.
Fabrication and characterization of nanoencapsulated cordyceps/turmeric extracts. (a) Nanoencapsulation and nanofabrication of CMP and TEP. (b) Size distributions were used nanoparticles tracking analysis system by Nanosight NS300. (c) The effect of cordycepin, CM, CMP, curcumin, TE and TEP on cell viability of SH-SY5Y cells post treatment. (d) The morphology of SH-SY5Y cells post treatment with a concentration (0.09 µM/day) of CMP or TEP. Data were presented as mean ± SD (n = 3) *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control cells.
Table 1.
Characterization of encapsulated nanoparticles by nanoparticles tracking analysis system using NanoSight NS300.
| Particles | CMP | TEP |
|---|---|---|
| Mean | 146.1 ± 1.2 nm | 195.2 ± 6.1 nm |
| SD* | 57.8 ± 4.6 nm | 83.9 ± 6.4 nm |
| Mode | 128.8 ± 3.1 nm | 130.1 ± 12.5 nm |
| Stock concentration (particles ml−1) | 1.46e + 009 | 7.58e + 008 |
*SD; width of the size distribution
The morphological changes of the treated SH-SY5Y cells were observed under an inverted microscope (Fig. 1d). First, the morphology of human neuroblastoma SH-SY5Y cells slightly changed by extending their cellular process after 24 h post-treatment of both 0.09 µM CMP and TEP. During day 1 and 3, some cell death was observed, but at day 5 & 7 of both treatments, the number of cell population was noticeably recovered. Interestingly, the cell arrangement of the treated cells at day 7 differed from the untreated cells with a long cell process and spindle shape. These results implied that CMP and TEP might influence cellular phenotypes of human neuroblastoma SH-SY5Y cells.
Nanoencapsulation Improved Absorption Efficiency of CM and TE
To evaluate the absorption efficiency and compare cellular uptake, human neuroblastoma SH-SY5Y cells were treated with 3 µM of CM, TE, CMP and TEP at different time points. The supernatant of treated cells was assessed the left-over concentration of cordycepin and curcumin to calculate the percentage of cellular uptake. Results showed that SH-SY5Y cells treated with CMP were able to absorb cordycepin up to 2.8 times, comparing with cordyceps medium in 6 h (Fig. 2a). While SH-SY5Y cells treated with TEP were able to absorb cordycepin up to 2.3 times, when compared with TE in 6 h (Fig. 2b). The absorption efficiency at cellular level of CM and TE was 22% and 9%, respectively, while the value of CMP and TEP was 71% and 75%, respectively (Fig. 2c). Notably, the prolonged treatment (5 days) presented the yellow colour of turmeric inside the cell sediment (Fig. 2d). Altogether, these results confirmed that nanoencapsulation increased the absorption efficiency of CM and TE into the cells, and might enhance their biological activity.
Figure 2.
Absorption efficacy of nanoencapsulated cordyceps/turmeric extracts on human neuroblastoma SH-SY5Y cells. (a, b) The absorption level of cordyceps medium, turmeric extract CMP and TEP on human neuroblastoma SH-SY5Y cells. (c) Comparison of percentage of cellular uptake between natural extracts and nanoparticles after treatment of human SH-SY5Y neuroblastoma cells at different intervals. (d) The cell sediment was collected and centrifuged after SH-SY5Y cells treatment. Data were presented as mean ± SD (n = 3) *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control cells.
Nanoencapsulated CMP and TEP Promoted Neuronal Maturation and Dopamine Secretion of Human Neuroblastoma SH-SY5Y Cells
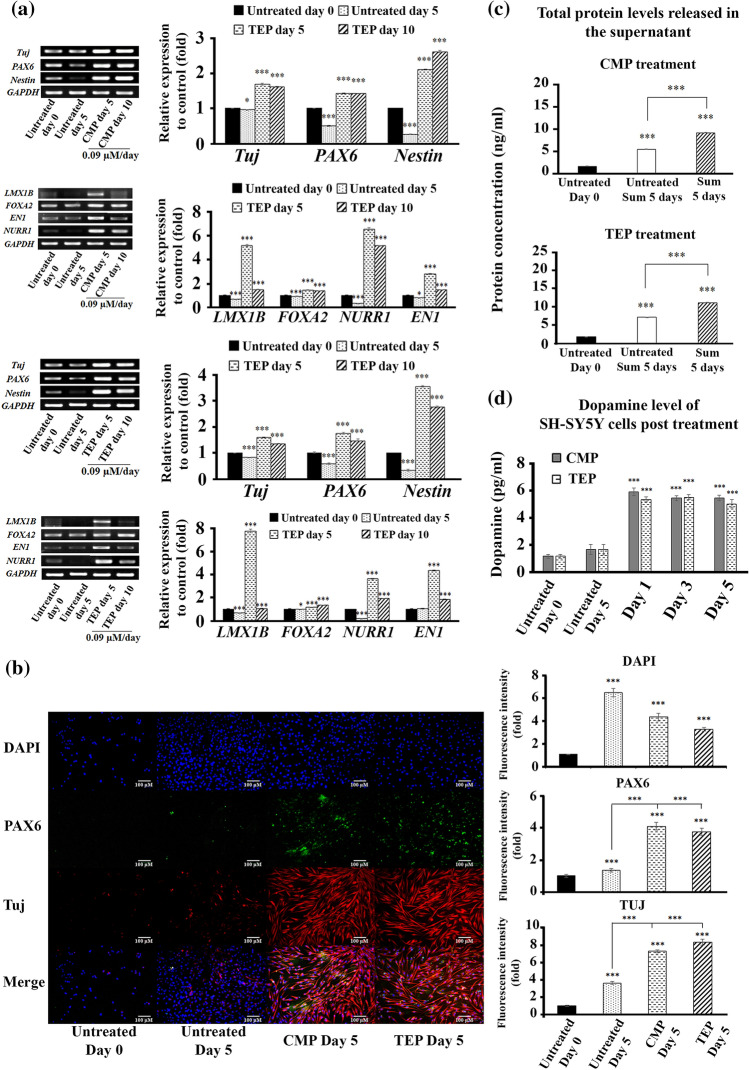
Since CMP and TEP affected the changes in cellular morphology, the nanoencapsulated products might also affect cellular function and maturation. To address this prospect, the expression of neuronal-specific genes was determined upon the treatment of CMP/TEP in human neuroblastoma SH-SY5Y cells. Upon the treatment of 0.09 µM CMP/TEP, the cells upregulated the expression of Tuj, PAX6, and Nestin genes up to 1.5–2.6 times and 1.2–3.4 times, respectively (Fig. 3a). The dopaminergic neuron-specific genes (LIM Homeobox Transcription Factor 1 Beta (LMX1B), Forkhead Box A1 (FOXA2), Engrailed Homeobox 1(EN1), and Nuclear receptor related 1 protein (NURR1)) were upsurged to 1.7–6.5 times (CMP) and 1.4–7.8 times (TEP), respectively after 5- and 10-day post treatment. Similarly, the expression of neuronal proteins, in particular Paired Box 6 (PAX6) and neuron-specific class III beta-tubulin (TUJ1), was intensified in human neuroblastoma SY-SY5Y cells after the treatment of 0.09 µM CMP/TEP. CMP and TEP activated PAX6 protein level by 4 times and 3.7 times, respectively, while TUJ1 protein was escalated 7.4 times by CMP and 8.4 times by TEP in human neuroblastoma SH-SY5Y cells (Fig. 3b). These results suggested that CMP and TEP could promote the maturation of human neuroblastoma SH-SY5Y cells.
Figure 3.
Neuronal maturation and dopamine secretion by human Neuroblastoma SH-SY5Y cells post treatment of CMP/TEP. (a) The expression of neuron genes (PAX6, TUJ, and Nestin), dopaminergic genes (LMX1B, FOXA2, EN1, and NURR1) of human neuroblastoma SH-SY5Y cells. The mRNA expression levels of genes relative to controls (fold) after SH-SY5Y cells were treated with a concentration (0.09 µM/day) of CMP or TEP. (b) The treated cells were staining using neuron-specific antibodies after 5 days post-treatment. (c) The accumulated level of protein secretion by human neuroblastoma SH-SY5Y cells post-treatment. (d)The dopamine secretion level was determined by competitive-ELISA assay using DA ELISA kit. Values were expressed as mean ± SD (n = 3). Significance vs. control cells is indicated as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
To further investigate the effects of CMP/TEP on neuronal function, the secretion of total protein and dopamine was assessed upon the treatment of CMP/TEP. The treated SH-SY5Y cells were measured for total protein secretion at day 5 post treatment using BCA assay. The results showed that the level of total protein secretion in the treated cells accumulated for 5 days was significantly increased, in which CMP increased 9 times, and TEP increased 11 times, compared to the untreated control cells (Fig. 3c). Next, dopamine secretion was evaluated by competitive-ELISA assay to ensure the neuronal-promoting effect of CMP and TEP. Human neuroblastoma SH-SY5Y cells were treated with 0.09 µM of CMP and TEP, and measured dopamine level on day 1, 3, and 5 post treatment. The level of secreting dopamine was significantly increased 3.3 times by CMP and 3.0 times by TEP, compare with the untreated control cells on day 5 (Fig. 3d). These results supported that the nanoencapsulated CMP and TEP promoted neuronal function of SH-SY5Y cells by accelerating neuronal maturation process.
Nanoencapsulated CMP and TEP Reduced the Expression of Alzheimer’s-Related Genes and the Secretion of Human Amyloid Precursor Protein
The three single-gene mutations associated with early-onset AD are Presenilin 1 (PSEN1), Presenilin 2 (PSEN2), and Amyloid precursor protein (APP). Oxidative stress is often associated with aging and neurodegenerative diseases. Substantial evidences commented that oxidative damage to the brain is an early event in AD. The natural substances with antioxidant properties may be responsible for reducing excess free radicals, and alleviating the pathology of AD. Nanoencapsulated CMP/TEP were examined their capacity to alleviate the expression of Alzheimer-related genes, including PSEN1, PSEN2, and APP. The expression of PSEN1, PSEN2, and APP was significantly reduced approximately 2–10 times, while the expression of the non-amyloidogenic pathway (ADAM Metallopeptidase Domain 10 (ADAM10) and Sirtuin1 (SIRT1)) was significantly increased upon the treatment of CMP/TEP (Fig. 4a). Next, the secretion of amyloid precursor protein was measured by sandwich-ELISA assay. Notably, the treatment of either 0.09 µM CMP or TEP for 5 days diminished the secretion of amyloid precursor protein 32-fold and 108-fold (Fig. 4b). These results presented that the nanoencapsulated CMP/TEP could improve the pathology of AD at both gene and protein levels.
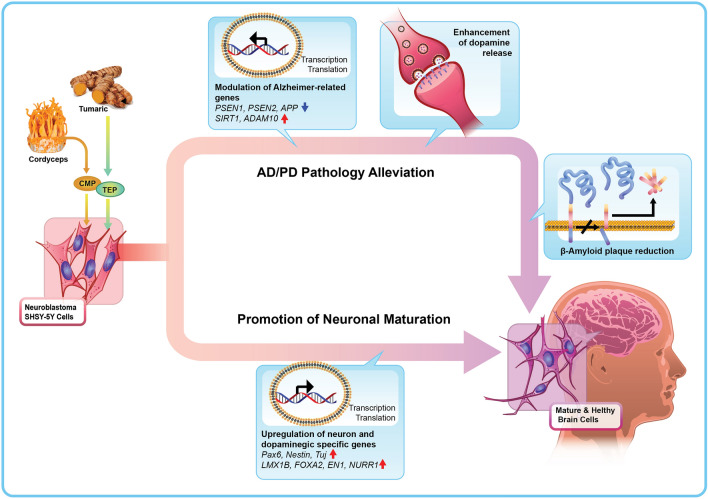
Figure 5.
Graphical illustration of the mechanism of actions of CMP/TEP to promote neuronal maturation and alleviate neurological pathology of human neuroblastoma SH-SY5Y cells.
Figure 4.
Expression of Alzheimer genes, APP level and autophagic activity of human neuroblastoma SH-SY5Y cells post-treatment of CMP/TEP. (a) The mRNA expression levels of Alzheimer genes relative to controls (fold) were determined after SH-SY5Y cells prolonged treatment. (b) The APP level was determined by sandwich-ELISA assay using Human APP Duo Set ELISA kit. (c) The treated cells were stained using autophagy marker anti-LC3 antibodies, and (d) labelling of autophagic vacuoles using MDC. The staining cells were observed under fluorescence microscope. Values were expressed as mean ± SD (n = 3). Significance vs. control cells was indicated as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Nanoencapsulated CMP and TEP Exerted Their Activity Through Autophagy Activation
It was known that the process of autophagy is important to neural differentiation and function; thus, nanoencapsulated CMP and TEP was assessed their involvement in autophagy activation. Immunofluorescences results showed that autophagy-related protein of microtubule-associated protein 1A/1B-light chain 3 (LC3) was intensified upon the treatment of 0.09 µM CMP and TEP at 2.3-folds and 1.9-folds, respectively (Fig. 4c). Similarly, MDC assay confirmed autophagy activation by CMP and TEP, in which the intensity of autophagic vacuole was significantly enhanced 6.3-folds by CMP and 5.5-folds by TEP, compared to untreated control cells (Fig. 4d). Altogether, these results highlighted that nanoencapsulated CMP/TEP could activate autophagy within human neuroblastoma SH-SY5Y cells, and this might lead to the improvement of neuronal condition of the cells.
Discussion
Our data presented the advantages of biodegradable nanopolymers from cassava starch to improve the neuropromoting effects of the nanoencapsulated products. CMP/TEP substantially enhanced the absorption efficiency and bioactivity of cordyceps and turmeric extracts. This highlighted the potential application of CMP/TEP to be used as an active ingredient in dietary supplements and cosmetic products. Importantly, CMP/TEP held a great promise as a therapeutic agent against neurological disorders, in particular AD, to relieve the production of amyloid precursor protein through autophagy activation. Nanoencapsulated products of cordyceps and turmeric extracts in this study contained the particle size smaller than their natural native form.2 This supported the high efficiency of both intracellular uptake and activation of targeted genes/proteins. The nanosuspension of modified cassava starch alone (negative control) was not toxic to neuroblastoma cells, but the death of the cells was caused by the natural extracts. Based on our results, the IC50 values of CMP and TEP were 0.94 and 1.31 uM, respectively, indicating that high dose administration affected apoptosis in neurons. However, it has been shown that taking lower doses of CMP and TEP can have a greater neuroprotective effect than CM and TE. This was consistent with the previous report that cordycepin induced apoptosis of human brain cancer cells through mitochondrial-mediated intrinsic pathway and the modulation of autophagy, while curcumin induces apoptosis in human neuroblastoma cells via inhibition of AKT and FOXO3a nuclear translocation.7,32 After nanoencapsulation, CMP and TEP increased their cellular absorption efficiency, which was similar to the water-soluble PLGA coated-curcumin nanoparticles.28 The modification of the surface, size, and shape is an important parameter to improve the therapeutic effects of nanoparticles, highlighting the significance of nanotechnology in advancing the physicochemical properties of nanoparticles in biomedical applications.39
One key finding here was that CMP/TEP could promote neuronal maturation and function of human neural SH-SY5Y cells. The treatments of CMP/TEP clearly enhanced the expression of pan-neuron genes (PAX6 and Nestin) and dopaminergic genes (LMX1B, FOXA2, EN1, NURR1). Curcumin significantly increased the brain dopamine levels,21 and also presented antidepressant effect by adjusting the release of serotonin and dopamine in the brain.22 Moreover, curcumin helped to prevent dopaminergic neuronal cell death through inhibition of the c-Jun N-terminal kinase pathway.51 On the other hand, cordycepin significantly improved dyskinesia associated with abnormal movement, and increased and maintained dopamine levels.14 PAX6 protein was found to increase by CMP/TEP, in which PAX6 functions in brain regional development and neuronal migration in the cerebral cortex. PAX6 acts is 2 steps; first, when a given progenitor leave the cell cycle, and second, the moment when a selected neuronal precursor irreversibly differentiates.27 Therefore, the on/off of PAX6 activity affects to neuronal differentiation in the developing brain and spinal cord.6 The neuron-specific class III beta-tubulin (TUJ1) protein is a neural progenitor marker, in which TUJ1 presents in newly generated immature postmitotic neurons and in some mitotically active neuronal precursors.45 Notably, the protein secretion level of the neuroblastoma cells was significantly increased upon the treatment of CMP/TEP. These results corresponded to the upsurged level of dopamine secretion by CMP/TEP treatment, validating the neuropromoting effects of those 2 nanoparticles.
Besides promoting neuronal maturation, CMP/TEP could alleviate the pathological hallmarks of Alzheimer’s disease. The expression of Alzheimer’s disease-related genes, including PSEN1, PSEN2, and APP, and the secretion of amyloid precursor protein from human neuroblastoma SH-SY5Y cells were decreased upon the treatment of CMP/TEP. Cordycepin was previously presented its neuroprotective effects to inhibit amyloid-β-induced apoptosis in hippocampal neurons,42 and protect against β–amyloid and ibotenic acid–induced hippocampal CA1 pyramidal neuronal hyperactivity.50 Similarly, curcumin inhibited Aβ aggregation and fibrillar Aβ formation,49 indicating that curcumin could protect neurons against Aβ toxicity.31 CMP/TEP was evidently activated autophagy within human neuroblastoma SH-SY5Y cells, marked by LC3 protein and MDC staining. Autophagy is an important protein degradation pathway in all cells, and particularly involves in cleansing Aβ aggresome formation within the brain cells.35 Autophagy can be considered as the intracellular center for the elimination of Aβ peptides and harmful Tau aggregates, emerging implication and treatment of AD.44 Recent observations suggested that the modulation of autophagy is related to AD progression. Regulating autophagy is connected to APP processing in AD, and, therefore, CMP/TEP might be applied to improve AD by regulating the activity of autophagy within the brain.34 Noteworthy, the enhanced efficacy of the active ingredients in combination with cassava starch nanopolymer may due to the nutritional value of cassava, containing numbers of nutritional compounds, such as proteins, amino acids, vitamins, and minerals. The studied substance involved in neural differentiation, neuroprotection, anti-apoptotic activity, cytotoxic activity, neurotransmission, neuronal maturation, including retinoic acid (vitamin A), thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), vitamin B6, folate (vitamin B9), vitamin C and vitamin K.37–46 Moreover, the roles of minerals, such as calcium, iron, magnesium, phosphorus, potassium and zinc, were also reported in neurodevelopment, prevention of cognitive deficits, AD progression, neurogenesis, and toxic responses of brain cancer.45–47
Conclusions
Taken together, we summarized the roles of nanoencapsulated cordyceps/turmeric extracts in promoting neuronal functions. The nanoencapsulated CMP and TEP were more effective than their natural form counterparts. CMP/TEP also synergized their antioxidant activity with the casava starch nanopolymer. Neurological diseases, including AD and PD, and brain cancers, are reportedly caused by genetic aberration and cellular malfunction. The aim of study is to develop the nanoencapsulated CMP and TEP, and investigate their capability against neurological pathology. It was found that both CMP and TEP could inhibit the proliferation of SH-SY5Y cells, and enhance the expression of mature neuronal markers. Importantly, upon the treatment of CMP and TEP the expression of dopaminergic-specific gene, and the secretion level of dopamine were significantly improved, while the expression of Alzheimer genes, and the secretion of amyloid precursor protein were significantly decreased. This finding suggested that the nanoencapsulated cordyceps and turmeric extracts could promote neuronal maturation, and alleviate neurological pathology in human neural cells.
Author Contributions
PK1, NS, and NC conceived and created the experimental design. NC, RP, and PK2 Contributed reagents/materials/analysis tools. PK1, NC and NS carried out the experiments. PK1, RP, and PN analysed and interpreted the results. PK1 and PK2 drafted the initial manuscript. PK1, PK2, and PN read, revised, and approved the final submitted manuscript.
Funding
This work was supported by Suranaree University of Technology (SUT), Thailand Science Research and Innovation (TSRI), and National Science, Research and Innovation Fund (NSRF) (project code 90464).
Conflict of interest
Palakorn Kaokaen (PK1), Natchadaporn Sorraksa (NS), Ruchee Phonchai (RP), Nipha Chaicharoenaudomrung (NC), Phongsakorn Kunhorm (PK2), and Parinya Noisa (PN) confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Human Subjects and Animal Study
No human or animals were used in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abd El-Hack ME, et al. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J Sci Food Agric. 2021;101:5747. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad M, et al. Nano-encapsulation of catechin in starch nanoparticles: characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019;270:95–104. doi: 10.1016/j.foodchem.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Aimo L, Oteiza PI. Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol Sci. 2006;91:184–191. doi: 10.1093/toxsci/kfj137. [DOI] [PubMed] [Google Scholar]
- 4.Allen GF, et al. Pyridoxal 5′-phosphate deficiency causes a loss of aromatic l-amino acid decarboxylase in patients and human neuroblastoma cells, implications for aromatic l-amino acid decarboxylase and vitamin B6 deficiency states. J Neurochem. 2010;114:87–96. doi: 10.1111/j.1471-4159.2010.06742.x. [DOI] [PubMed] [Google Scholar]
- 5.Arslan ME, Turkez H, Mardinoglu A. In vitro neuroprotective effects of farnesene sesquiterpene on alzheimer's disease model of differentiated neuroblastoma cell line. Int J Neurosci. 2021;131:745–754. doi: 10.1080/00207454.2020.1754211. [DOI] [PubMed] [Google Scholar]
- 6.Bel-Vialar S, Medevielle F, Pituello F. The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord. Dev Biol. 2007;305:659–673. doi: 10.1016/j.ydbio.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Chaicharoenaudomrung N, Jaroonwitchawan T, Noisa P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol In Vitro. 2018;46:113–121. doi: 10.1016/j.tiv.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 8.French AE, et al. Folic acid food fortification is associated with a decline in neuroblastoma. Clin Pharmacol Therapeut. 2003;74:288–294. doi: 10.1016/S0009-9236(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 9.Galetta KM, Bhattacharyya S. Multiple sclerosis and autoimmune neurology of the central nervous system. Medical Clinics. 2019;103:325–336. doi: 10.1016/j.mcna.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Gera M, et al. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget. 2017;8:66680–66698. doi: 10.18632/oncotarget.19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goranov B, Hewson QC, Pearson A, Redfern C. Overexpression of RAR γ increases death of SH-SY5Y neuroblastoma cells in response to retinoic acid but not fenretinide. Cell Death Differ. 2006;13:676–679. doi: 10.1038/sj.cdd.4401824. [DOI] [PubMed] [Google Scholar]
- 12.He XB, et al. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET 1-and JMJD 3-dependent epigenetic control manner. Stem cells. 2015;33:1320–1332. doi: 10.1002/stem.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiboonma A, et al. Cordycepin attenuates salivary hypofunction through the prevention of oxidative stress in human submandibular gland cells. Int J Med Sci. 2020;17:1733–1743. doi: 10.7150/ijms.46707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, et al. Cordycepin exerts neuroprotective effects via an anti-apoptotic mechanism based on the mitochondrial pathway in a rotenone-induced parkinsonism rat model. CNS Neurol Disord Drug Targets. 2019;18:609–620. doi: 10.2174/1871527318666190905152138. [DOI] [PubMed] [Google Scholar]
- 15.Joye IJ, McClements DJJTIFS. Technology production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci Technol. 2013;34:109–123. doi: 10.1016/j.tifs.2013.10.002. [DOI] [Google Scholar]
- 16.Juna S, Hayden S, Damm M, Kappe CO, Huber AJSS. Microwave mediated preparation of nanoparticles from wx corn starch employing nanoprecipitation. Starch. 2014;66:316–325. doi: 10.1002/star.201300067. [DOI] [Google Scholar]
- 17.Kaokaen P, et al. Cordycepin-loaded nanoparticles from cassava starch promote the proliferation of submandibular gland cells and inhibit the growth of oral squamous carcinoma cells. Nutr Cancer. 2020;1:1–16. doi: 10.1080/01635581.2020.1819350. [DOI] [PubMed] [Google Scholar]
- 18.Kitano T, et al. Vitamin K3 analogs induce selective tumor cytotoxicity in neuroblastoma. Biol Pharm Bull. 2012;35:617–623. doi: 10.1248/bpb.35.617. [DOI] [PubMed] [Google Scholar]
- 19.Kocot J, Luchowska-Kocot D, Kiełczykowska M, Musik I, Kurzepa J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients. 2017;9:659. doi: 10.3390/nu9070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovalevich J, Langford D. Neuronal Cell Culture. Berlin: Springer; 2013. pp. 9–21. [Google Scholar]
- 21.Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology. 2008;201:435. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni S, Dhir A. An overview of curcumin in neurological disorders. Indian J Pharm Sci. 2010;72:149. doi: 10.4103/0250-474X.65012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunhorm P, Chaicharoenaudomrung N, Noisa P. Enrichment of cordycepin for cosmeceutical applications: culture systems and strategies. Appl Microbiol Biotechnol. 2019;103:1681–1691. doi: 10.1007/s00253-019-09623-3. [DOI] [PubMed] [Google Scholar]
- 24.Lemming, M.T., Hoynowski, S.M., Xu, Y. & Mitchell, K.E. (Wiley Online Library, 2008).
- 25.Liu G. Prevention of cognitive deficits in Alzheimer’s mouse model by elevating brain magnesium. Mol Neurodegen. 2012;7:1–1. doi: 10.1186/1750-1326-7-S1-L24. [DOI] [Google Scholar]
- 26.Liu J, et al. Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner. Int J Nanomed. 2017;12:8085. doi: 10.2147/IJN.S149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manuel MN, Mi D, Mason JO, Price DJ. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front Cell Neurosci. 2015;9:70. doi: 10.3389/fncel.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathew A, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer's disease. PLoS ONE. 2012;7:e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood JJCSR. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941–3994. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- 30.Oberbauer E, et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen. 2015;4:7. doi: 10.1186/s13619-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S-Y, Kim DS. Discovery of natural products from Curcuma l onga that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer's disease. J Nat Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 32.Picone P, et al. Curcumin induces apoptosis in human neuroblastoma cells via inhibition of AKT and Foxo3a nuclear translocation. Free Radic Res. 2014;48:1397–1408. doi: 10.3109/10715762.2014.960410. [DOI] [PubMed] [Google Scholar]
- 33.Påhlman S, Ruusala A-I, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14:135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 34.Rahman M, et al. Modulatory effects of autophagy on APP processing as a potential treatment target for Alzheimer’s disease. Biomedicines. 2021;9:5. doi: 10.3390/biomedicines9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman MA, et al. Autophagy modulation in aggresome formation: emerging implications and treatments of Alzheimer's disease. Biomedicines. 2022;10:1. doi: 10.3390/biomedicines10051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakshit J, et al. Iron-induced apoptotic cell death and autophagy dysfunction in human neuroblastoma cell line SH-SY5Y. Biol Trace Element Res. 2020;193:138–151. doi: 10.1007/s12011-019-01679-6. [DOI] [PubMed] [Google Scholar]
- 37.Reed X, Bandres-Ciga S, Blauwendraat C, Cookson MR. The role of monogenic genes in idiopathic Parkinson's disease. Neurobiol Dis. 2019;124:230–239. doi: 10.1016/j.nbd.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakarcan S, et al. Riboflavin treatment reduces apoptosis and oxidative DNA damage in a rat spinal cord injury model. Clin Exp Health Sci. 2017;7:55–63. doi: 10.5152/clinexphealthsci.2017.218. [DOI] [Google Scholar]
- 39.Salatin S, Maleki Dizaj S, Yari Khosroushahi A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int. 2015;39:881–890. doi: 10.1002/cbin.10459. [DOI] [PubMed] [Google Scholar]
- 40.Satheesh NJ, Büsselberg D. The role of intracellular calcium for the development and treatment of neuroblastoma. Cancers. 2015;7:823–848. doi: 10.3390/cancers7020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sdek P, et al. Alteration of cell-cycle regulatory proteins in human oral epithelial cells immortalized by HPV16 E6 and E7. Int J Oral Maxillofac Surg. 2006;35:653–657. doi: 10.1016/j.ijom.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Song H, et al. Neuroprotective effects of cordycepin inhibit Aβ-induced apoptosis in hippocampal neurons. Neurotoxicology. 2018;68:73–80. doi: 10.1016/j.neuro.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, et al. Fabrication of size-controlled starch-based nanospheres by nanoprecipitation. ACS Appl Mater Interface. 2009;1:956–959. doi: 10.1021/am900054f. [DOI] [PubMed] [Google Scholar]
- 44.Tung Y-T, et al. Autophagy: a double-edged sword in Alzheimer’s disease. J Biosci. 2012;37:157–165. doi: 10.1007/s12038-011-9176-0. [DOI] [PubMed] [Google Scholar]
- 45.Und Halbach OVB. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329:409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, et al. Thiamine deficiency induces endoplasmic reticulum stress in neurons. Neuroscience. 2007;144:1045–1056. doi: 10.1016/j.neuroscience.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasiak T, et al. Phosphorus dendrimers affect Alzheimer’s (Aβ1–28) peptide and MAP-Tau protein aggregation. Mol Pharm. 2012;9:458–469. doi: 10.1021/mp2005627. [DOI] [PubMed] [Google Scholar]
- 48.Xie H-R, Hu L-S, Li G-Y. SH-SY5Y human neuroblastoma cell line: in vitrocell model of dopaminergic neurons in Parkinson's disease. Chin Med J. 2010;123:1086–1092. [PubMed] [Google Scholar]
- 49.Yang F, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 50.Yao L-H, et al. Cordycepin protects against β-amyloid and ibotenic acid-induced hippocampal CA1 pyramidal neuronal hyperactivity. Korean J Physiol Pharmacol. 2019;23:483–491. doi: 10.4196/kjpp.2019.23.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu S, et al. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenat Res. 2010;13:55–64. doi: 10.1089/rej.2009.0908. [DOI] [PubMed] [Google Scholar]