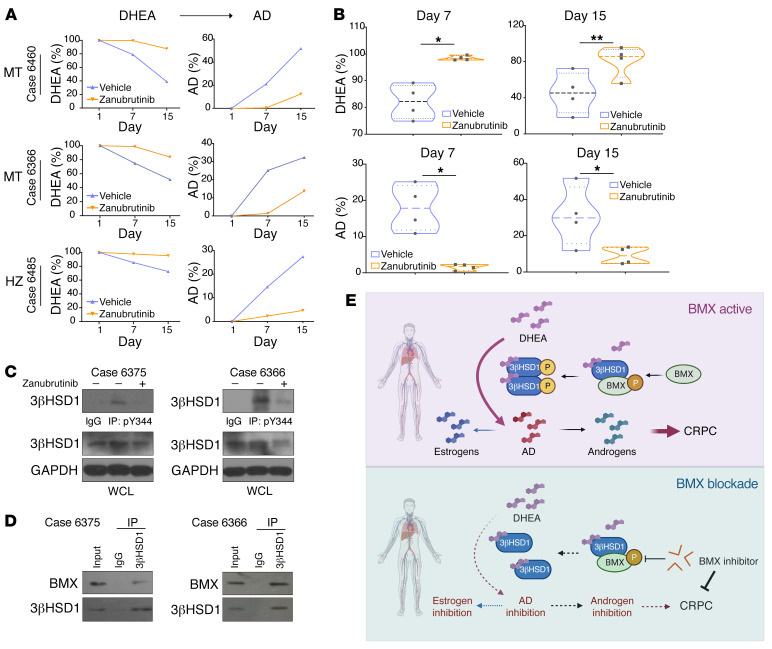
Figure 6. Targeting BMX inhibits phosphorylation and enzymatic activity of 3βHSD1 in prostate tissue of prostate cancer patients.
(A) Fresh prostate tissues from 3 representative patients with prostate cancer from a total of 7 patients in whom DHEA metabolism was detectable; DHEA metabolism was analyzed in a total of 42 patients (MT, homozygous HSD3B1(1245C); HZ, heterozygous). Tissues were obtained and aliquoted in 2 equal portions. 1 portion was treated with zanubrutinib and the other with DMSO. Both portions were maintained in 3 ml DMEM containing 10% FBS, incubated for 12 hours, and then [3H]-DHEA was added to each portion. Cell culture medium was collected at the indicated times, and HPLC was performed. (B) DHEA metabolism was analyzed on day 7 and day 15. Mean ± SEM represents DHEA metabolism from 4 patients. *P < 0.05, **P < 0.01 (unpaired 2-tailed t test). (C) Protein was extracted from about 20 mg patient tissue, followed by 3βHSD1 immunoprecipitation and Western blot. (D) The remaining tissue was used for Western blot: tissue cores were minced and aliquoted in 2 equal parts and treated as in A. After 12 hours of culture, DHEA (10 nM) was added to each portion. 7 days later, protein was collected, and immunoprecipitation and Western blot were performed. (E) Proposed model for 3βHSD1 phosphorylation. BMX phosphorylates 3βHSD1 Y344 upon activation by DHEA. Y344 phosphorylation enhances 3βHSD1 activity by increasing its dimerization, which subsequently promotes androgen production and prostate cancer proliferation. When BMX is inhibited, 3βHSD1 phosphorylation–stimulated dimerization is lost, reducing cellular enzyme activity, potent androgen production, and prostate cancer proliferation. 3βHSD1 inhibition also blocks estrogen synthesis. For all panels, error bars represent the SEM; P values were calculated using paired 2-tailed t tests.