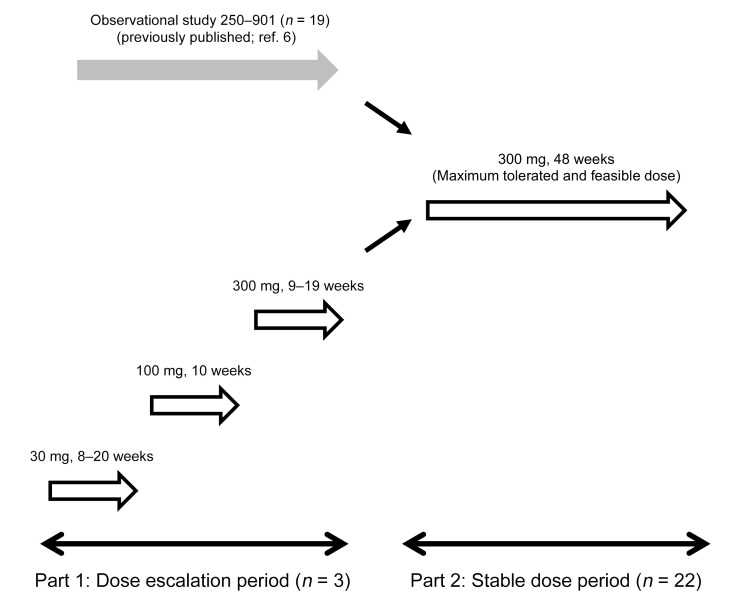
Figure 1. Flow diagram of Study 250-201.
In Part 1, 3 patients, i.e., 9001, 9002, and 9003, were recruited and treated with escalating doses of 30, 100, and 300 mg tralesinidase alfa as described in Results. These 3 patients were eventually recruited to Part 2 of the study and treated for an additional 48 weeks. In addition, 19 patients, i.e., patients 9004–9015 and 9017–9023, previously observed in our natural history Study 250-901 (6), were treated with 300 mg tralesinidase alfa. One patient, 9016, withdrew from the trial prior to the first drug administration. In all cases, treatment was done weekly through i.c.v. administration.