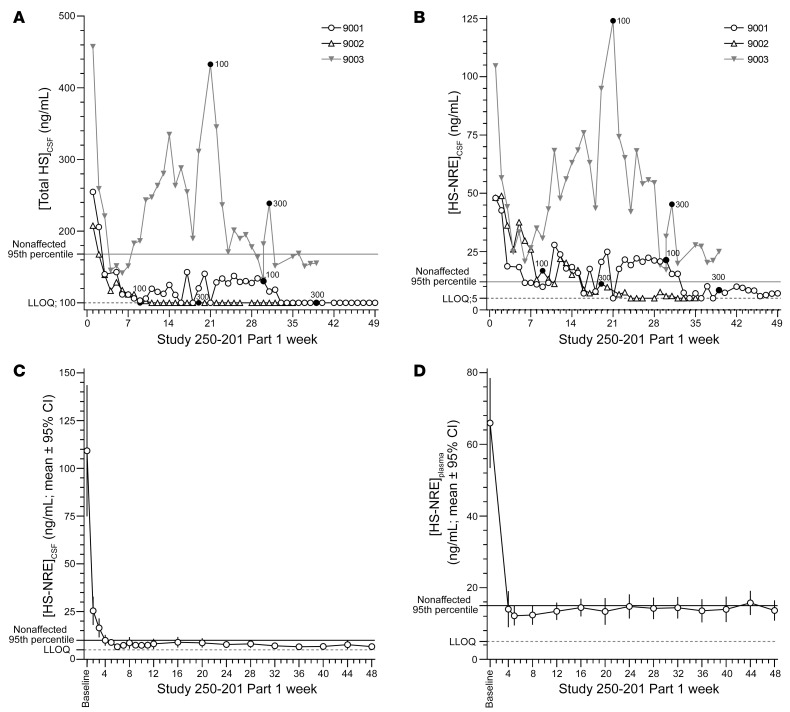
Figure 3. A 300 mg dose of tralesinidase alfa administered weekly i.c.v. normalizes total HS and HS-NRE levels in the CSF and plasma.
In Study 250-201 Part 1, three patients, i.e., 9001, 9002, and 9003, were treated with 30, 100, or 300 mg tralesinidase alfa weekly. Total HS (A) and HS-NRE (B) concentrations were quantified weekly in the CSF of treated patients using a Sensi-Pro assay as described in Methods. Black dots labeled 100 or 300 indicate the weeks when treatment increased for each patient from 30 to 100 mg or from 100 to 300 mg. In Study 250-201 Part 2, the patients (n = 22) were treated weekly for 48 weeks with 300 mg tralesinidase alfa. HS-NRE was quantified in the CSF (C) and plasma (D) of treated patients weekly or at least every 4 weeks using the Sensi-Pro assay as described in Methods. Two patients were excluded from the data in C and D because they received only 17% and 57%, respectively, of the tralesinidase alfa doses expected to be administered from baseline to week 48 of Study 250-201. Data in C and D are expressed as the mean ± 95% CI. Supplemental Tables 1 and 3 list the individual values for each data point and each patient.